Disposables 14995
October 15, 2004
Originally Published MPMN October 2004
SPOTLIGHT
Disposables
IV fluid control
Product design and development is available from a provider of IV fluid control technology. Projects are followed from concept to market by developing required technologies and devising strategic business solutions. The company specializes in one-way and bidirectional check valves as well as needleless access systems. Manufacturing is performed in Class 100,000 cleanrooms. Nypro Medical Products Group, Clinton, MA www.npmedical.com
Flexible catheter systems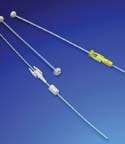
A maker of custom medical devices and components manufactures complex disposables for a catheter system used for high- and pulsed-dose cancer treatments. The treatment needle assembly can be removed between patient treatments. The catheter buttons and needle assemblies are marked with channel numbers for quick, accurate reconnection to the system’s afterloaders. The system design reduces the chance of catheter kinking. Medisize, Hillegom, Netherlands www.medisize.com
Liquid dispenser 
Cleaning validation of a liquid-dispensing system is simplified with peristaltic pump technology. Disposing of the entire fluid path eliminates cross-contamination between production runs. For each production batch, a new, sterile, disposable tube is used. In most cases, product changeovers and validation can be accomplished in less than 5 minutes. The company’s liquid-filling technology is suitable for pilot lot manufacturing as well as large-scale filling operations. Flexicon America Inc., Burlington, VT www.flexiconamerica.com
Adhesive materials and manufacturing
Three adhesive coating lines produce a variety of single- and double-coated pressure-sensitive materials. Typical materials include woven and nonwoven fabrics, plastic and breathable films, foams, foils, and paper. Adhesion characteristics can be targeted to meet specific product performance requirements. The company also offers slitting, sheeting, die-cutting, and packaging services. Kapco, Kent, OH www.kapco.com
Miniature screened orifice
Consistent, uniform flow is an essential requirement of medical fluid-handling systems and components. A miniature screened orifice is designed for these types of critical flow control applications. The orifice is preinstalled in a male-to-female luer adaptor and features an integral safety screen that protects the device from contamination. Designed for both liquid and gas media, the orifice is available in 18 standard flow rates. Each part is 100% tested with either water or nitrogen to ensure 5% flow-rate tolerances. The orifice is made from stainless steel and the luer adaptor is made of medical-grade polypropylene. The Lee Co., Westbrook, CT www.theleeco.com
Needle-free valves
Needle-free valves feature a flush, swabbable top to minimize contamination. Made of latex-free materials, the SmartSite valves comprise only three parts and have a straight fluid-path design similar to a standard injection port. The streamlined design makes the valves cost-effective to manufacture. SmartSite technology can be adapted to most IV administration applications. Uses include infusion pump sets, heparin and saline locks, extension sets, vial access pins, vial adapters, and bag spikes. Alaris Medical Systems, San Diego, CA www.alarismed.com
Disposable tubing and molded fittings
Extruded, corrugated, and collapsible plastic tubing and injection-molded fittings are available from a manufacturer. Production and assembly services are also offered. Visitors to the company’s Web site can view images of its product lines and request samples. GlobalMed Inc., Trenton, ON, Canada www.globalmedinc.com
Die-cutting, laminating, and slitting services
A manufacturer specializes in precision die-cutting, laminating, slitting, rewinding, assembly, and printing. Custom fabrications are built to customer specifications. The company can convert different materials, including medical-grade tapes and films, foils, papers, and hydrogels. Assistance in materials selection and design is also offered. Pepin Manufacturing Inc., Lake City, MN www.pepinmfg.com
Copyright ©2004 Medical Product Manufacturing News
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
