Tough Medicare Reimbursement Proposal for Watchman
A requirement in Medicare's proposed decision memo on coverage for Boston Scientific's Watchman conflicts with FDA's approved indication for the device. What now?
November 11, 2015
Marie Thibault
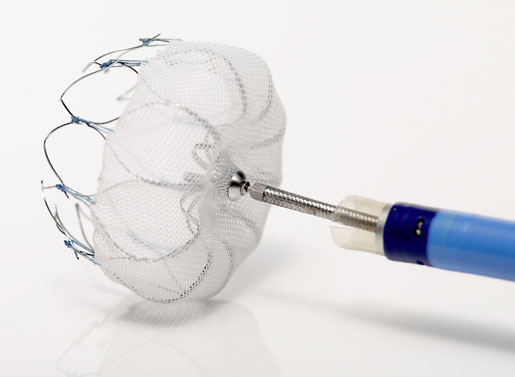
The Watchman left atrial appendage closure (LAAC) device had a bumpy road to approval and it looks like its journey toward Medicare reimbursement may not be any easier. In April of this year, Boston Scientific submitted a formal request to CMS for a National Coverage Determination. On Tuesday, CMS released its proposed decision memo from its national coverage analysis for percutaneous LAAC therapy, calling for use of its Coverage with Evidence Development (CED) approach.
CMS wrote that it believes "the evidence is sufficient to determine percutaneous LAAC therapy using an implanted device is not reasonable and necessary to diagnose or treat an illness or injury or to improve the functioning of a malformed body member and, therefore, is not covered . . . " The proposal to follow the CED paradigm would require use of Watchman to meet several criteria in order to quality for reimbursement coverage.
The biggest issue with these criteria is the requirement that the Watchman patient have a contraindication to warfarin. In the FDA approval from March 2015, the agency made it clear that the device should only be used in patients who "are suitable for warfarin."
The requirements, detailed in the CMS proposed decision memo, include:
FDA approval of the device for use in non-valvular atrial fibrillation patients
Patients must have a high stroke risk score, a high major bleed risk score, and a contraindication to warfarin
The procedure is done in a hospital with key capabilities and equipment, like a cardiac catheterization or electrophysiology lab with fluoroscopy, non-invasive imaging with echocardiography support, anesthesiology support, and emergency cardiac surgery services
The primary implanting physician, who must be an interventional cardiologist and/or an electrophysiologist, must have performed 25 or more trans-septal puncture procedures, with at least 10 such procedure in the last year
The primary implanting physician needs to undergo training on safe and effective use from the manufacturer, as well as training from a physician who has completed cases. This must include at least two supervised and two observed procedures.
The physician team must participate in and the patient must be enrolled in a prospective national registry evaluating LAAC patients and specific outcomes annually for at least five yeara post-procedure.
Documentation of a formal shared decision-making interaction between the patient and provider must be made.
The proposed decision memo is open to public comment for 30 days.
Joanne Wuensch, analyst at BMO Capital Markets, wrote in a research note Wednesday that the warfarin language "is in direct contradiction to the FDA label, would significantly narrow the potential patient population, and needs to be better understood."
In optimistic commentary, Wells Fargo senior analyst Larry Biegelsen wrote in a research note Wednesday, "The proposed NCD contains requirements that make it almost impossible to receive reimbursement for Watchman which we do not believe was CMS’s intent. We expect an outpouring of pressure from the key medical societies during the 30 day comment period which will likely lead to CMS amending the NCD when it issues the final version which is scheduled for release by 2/8/16."
In an email to MD+DI, a Boston Scientific spokesperson wrote, "The proposed decision notes CMS will cover percutaneous LAAC therapy for patients with non-valvular atrial fibrillation when seven conditions are met. These proposed conditions are similar to conditions under which other new technologies, such as TAVR and TMVR, have been granted coverage. Boston Scientific is working to better understand the proposed decision and seek appropriate clarification. We expect CMS to finalize the decision in February 2016 after review of public comments which must be submitted within 30 days."
Check out the future of medical technology—register for the BIOMEDevice San Jose Conference, December 2-3, 2015. |
Marie Thibault is the associate editor at MD+DI. Reach her at [email protected] and on Twitter @medtechmarie.
[Image courtesy of BOSTON SCIENTIFIC CORP.]
About the Author(s)
You May Also Like


