Putting Implants to the Test
Many tools and processes are used to check implant safety and performance.
May 1, 2008
TESTING
|
Lumetrics's OptiGauge noncontact system uses reflected light from the probe to measure all layers of this breast implant. |
Like other products, implantable medical devices are tested to determine whether they perform their intended functions and how well they do so. But unlike other products, medical implants are also tested to see whether they can somehow cause harm during long-term residence in the human body.
To satisfy government agencies and meet international requirements, implants undergo many different safety tests spanning a variety of technologies and requiring widely varying amounts of time and money.
How best to meet this testing challenge? The answer depends on a number of factors, including the implant maker's in-house capabilities, testing workload, and confidentiality concerns.
Evaluating Biocompatibility
FDA's main concern is safety, so much of the testing done on new products seeks to evaluate potential risks.
For guidance on many types of safety testing, implant makers turn to ISO 10993, which contains a series of assays for evaluating the biocompatibility of a medical device. This job includes the chemical characterization of materials, which is covered in Part 18 of 10993. When characterizing an implant material, “it's not enough to analyze the surface. You have to know what's going to leach out of the material into the tissue environment,” notes Anne Schuler. She is quality assurance manager for LexaMed Ltd. (Toledo, OH), which provides laboratory services to the medical device industry.
To find out what will leach out of an implant material, Schuler explains, lab personnel perform physical chemical tests from the United States Pharmacopeia. The first step is to expose the material to an environment similar to that inside the body for an extended period of time. Thus the material is soaked in a solvent (for example, water or alcohol) or a lipid that simulates body fat at 37°C, which is normal body temperature.
Then a variety of chemical tests are run on the extract from the soaking process, which includes whatever substances leached out of the material. The chemical procedures include gas chromatography (GC) and high-performance liquid chromatography (HPLC) tests, as well as the inductively coupled plasma assay. These tests identify the specific organic and inorganic materials in the extract.
Once this information has been obtained, Schuler says, the medical device manufacturer must determine whether the amounts of the substances found in the extract are at acceptable levels. This is done by performing a risk assessment that includes a search of the available literature on the material in question. A key factor in assessing risk is how the implant material will be used in the body, she notes.
The equipment needed to perform tests such as GC and HPLC can cost hundreds of thousands of dollars. Rather than buying this equipment, Schuler says, “it's probably more cost-effective for manufacturers to farm out chemical testing to contract research organizations, especially if they're only making one or two devices that require it.”
Biological Procedures
Generally speaking, chemical testing is more important in Europe than in the United States, says Larry Lister, director of biocompatibility for Toxikon Corp. (Bedford, MA), which provides testing services to the medical device industry. In the United States, Lister says, “biological testing is the real safety testing. We want to see whether a material is compatible with tissue. Even if chemical analysis shows that nothing [harmful] is coming out of a material, we want to make sure that it's biologically safe.”
Like chemical testing, some biological testing is done using extracts. These are obtained by immersing test materials in saline (which represents human blood) or vegetable oil (which represents body fat) and baking the materials for different periods of time at temperatures ranging from 37° to 121°C, depending on the material. “The idea is to get whatever leaches out of the material under those conditions, which are meant to exaggerate the conditions” an implant will experience in the body, Lister explains.
|
Here, the OptiGuage system measures the coating thickness and uniformity on a stent. |
In some biological tests, animals or cells are exposed to extracts from this process, which include any substances that leached out of the test material. In others, the material itself is implanted into animals. Biological tests in ISO 10993 are outlined below.
Genotoxicity (10993-3). This test looks for evidence that substances leaching from an implant can damage cells involved in reproduction. If a substance can damage the DNA of such cells, it can potentially alter DNA in normal tissue cells, which can result in cancer, notes Joseph Carraway, director of toxicology for North American Science Associates Inc. (NAMSA; Northwood, OH). NAMSA specializes in the safety evaluation of medical devices and materials.
The test is performed by exposing different types of cells to extracts. If the cells or cell colonies multiply, that could indicate the presence of a genotoxic material.
When doing genotoxicity testing, Carraway says, Europeans strictly follow ISO guidelines, which limit the required tests to in vitro assays. But FDA subscribes to slightly different guidelines that require inclusion of an in vivo assay.
Carcinogenicity (10993-3). Carcinogenicity testing is required when genotoxicity tests yield positive results and when new materials are used for implants. The procedure is done by implanting the test material in rodents, which are exposed to 100 times more material per unit body weight than a human would be in order to provide a safety factor in the testing. Until recently, Carraway says, carcinogenicity testing required lab personnel to study large numbers of rodents for their entire lives—typically 2 years or more. But now the test can be performed on transgenic mice that are genetically altered to be more sensitive to carcinogens, which cuts the required testing time down to 6 months. At the end of that time, personnel compare the number and type of tumors found in the test animals to what is found in a control group.
Hemocompatibility (10993-4). In this test blood is exposed to extracts or to the test material to see whether it causes blood cells to rupture or changes the way the blood clots.
In Vitro Cytotoxicity (10993-5). To assess cytotoxicity, cells grown on plates are exposed to an extract to determine whether a material is toxic to the cells.
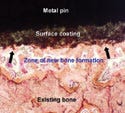
Implantation (10993-6). To determine whether implanted material is having any local effects, both gross visual and microscopic examinations of host animals are made to assess the reaction at the implant site at different points in time—for example, at 4 weeks to see the early reaction, then at 3 months, and finally at 6 months to a year to discover the longer-term reaction. Among the things lab personnel look for are cells attracted to the implanted material, rejection of the material, and tissue death at the implant site.
Irritation (10993-10). An extract is injected into the skin of animals to see if it causes irritation.
Sensitization (10993-10). In this assay, guinea pigs are repeatedly exposed to an extract to determine whether it causes an allergic response. No level of sensitization is acceptable, Lister notes.
Systemic Toxicity (10993-11). According to Carraway, virtually all implantable devices require systemic toxicity testing, which determines whether leachables from a device can produce a systemic effect, as opposed to the local effects checked for during implantation testing. Most implantables require both subchronic
(1–3 months) and chronic (6 months or longer) assessments, he adds, although one or the other may be avoided depending on the implant's duration in the body.
Systemic toxicity testing is usually done by implanting parts of a device in rodents. During the test period, laboratory personnel look for evidence of illness and weigh the rodents at regular intervals. At the end of the study, blood, tissues, and organs of the rodents are microscopically checked for damage.
Breast Implant Testing |
To determine which tests must be run for a certain implant, Lister says, medical device manufacturers consult testing matrices such as the one in ISO 10993-1. Other matrices are published by FDA and Japan's Ministry of Health, Labour, and Welfare. The matrices tell manufacturers which tests to perform based on what an implant contacts inside the body and how long that contact is maintained, Lister explains.
The cost of biological testing ranges from several hundred dollars to hundreds of thousands of dollars, depending on factors such as the duration of the test and the type and number of animals involved. Testing can take anywhere from a couple of weeks to a couple of years. Fortunately for implant manufacturers, tests that last for years are rarely required, says Gary Swanson, president of Geneva Laboratories Inc. (Elkhorn, WI), which provides testing services to the medical industry. According to Swanson, the worst-case testing scenarios involve implants made of new materials that are going into the bloodstream.
Who Does the Job?
Most biological testing is done by contract laboratories, according to Carraway, who contends that results from contract labs may carry more weight with FDA and other regulatory bodies than results of in-house testing done by medical device manufacturers. Why? “When the testing is done by an outside party, that party doesn't have a vested interest in the outcome of the test,” he notes.
On the other hand, some companies may want to keep biological testing in-house “to keep control of the information,” says Swanson. “If you have a unique material, this can be an advantage. You know you have control of the test data if the testing is done in your company-owned facility.”
Lister reports that a number of large medical device manufacturers do biological testing at their own facilities and outsource whatever testing work they can't handle. But it's a costly proposition to maintain such facilities, which require a group of scientists, a quality assurance unit, and an animal-husbandry staff.
“There's a lot involved in maintaining a GLP animal facility,” Lister notes. “So if your business is making bone screws, it's really not cost-effective for you to have a staff dedicated to animal testing that's only required once for a product.”
Other Safety Testing
|
(click to enlarge) |
Some of the necessary safety testing provides information about critical mechanical properties of products. Such properties include the radial strength and stiffness of stents, which must be measured to meet FDA requirements. Machine Solutions Inc. (Flagstaff, AZ) offers a system that can be used to perform such tests.
This stent testing “is generally done in-house by major manufacturers,” explains Melissa Lachowitzer, the firm's testing product manager. “Some of the smaller ones will farm it out. They'll use our services for a time as they're getting their first device onto the market. But once they have an approved device, most of them will purchase the equipment.” To buy the equipment, it will cost a firm about $50,000. To use Machine Solutions's contract services, for example, it costs about $3000 to test 20 devices and produce a report of the results.
The firm's system measures the amount of outward force a stent exerts on the inner wall of a vessel, as well as the amount of force required to collapse the stent. By doing so, the device “makes sure a stent is strong enough to hold its position in the body, but not so strong that it's going to damage a vessel,” says Lachowitzer.
The system includes a chamber in the shape of a 12-sided polygon, which holds the stent to be tested. The shape of the chamber allows it to compress the stent at 12 points equally spaced around its perimeter, while the system measures the amount of force required to compress the stent. Then, as the chamber opens up again, the system measures the force exerted on it by the stent. (An article on radial strength and stiffness testing written by Lachowitzer can be found here.)
The stents are tested at body temperature but not in liquid, so users can't determine how the forces in question will be affected when a stent is exposed to body fluids. “We're not sure how much [this limitation] affects the test results, but I think it's fairly minimal,” Lachowitzer says.
Conclusion
Whether it's done in-house or at a contract lab, testing yields valuable information about implantable medical devices. During research and development, test data can be used to help manufacturers assess the quality and functionality of implants. In addition, data from a variety of chemical and biological tests satisfy safety requirements meant to minimize the chances that implants will harm the patients they are intended to help.
Copyright ©2008 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
