Pulmonx Closing in On Approval for Valve to Treat Emphysema
Pulmonx released positive one-year-results from the LIBERATE pivotal trial of the Zephyr Endobronchial Valve, which shows the study met all primary and secondary endpoints.
May 24, 2018
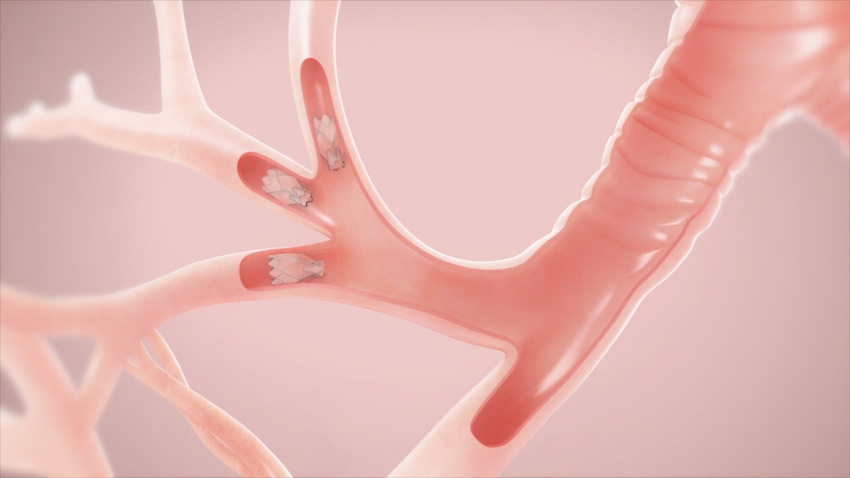
Pulmonx has some additional ammunition in its bid to get its severe emphysema treatment valve FDA approval. The Redwood City, CA-based company released positive one-year-results from the LIBERATE pivotal trial of the Zephyr Endobronchial Valve, which shows the study met all primary and secondary endpoints in treating emphysema patients.
Emphysema falls under the umbrella of chronic obstructive pulmonary disease (COPD) and is characterized by damage to the walls of the lungs between the air sacs, according to the National Emphysema Foundation. This damage causes the sacs to lose their shape and often, the destruction of those walls, leading to fewer and larger air sacs instead of many tiny ones. As a result, the amount of gas exchanged in the lungs is reduced and patients have difficulty breathing.
“We put in our filing with FDA at the very end of last year,” Beran Rose, VP of Marketing and Business Development, Pulmonx, told MD+DI. “The [filing] was a particularly strong package because it included not only the data from our U.S. pivotal study, but also the data from four prior studies done with this device. We’re really excited about the prospect of commercializing this technology in the U.S. should FDA approve it, and we’re hoping that may be sometime in the second half of this year.”
LIBERATE evaluated the effectiveness and safety of the Zephyr Endobronchial Valve out to one year in patients with severe heterogeneous emphysema with little to no collateral ventilation in the target lobe of the lung. The study randomized 190 patients at 24 sites, using a 2:1 randomization to Zephyr Valve treatment or medical management alone.
The LIBERATE Study results were concurrently published in the American Journal of Respiratory and Critical Care Medicine and presented at the American Thoracic Society 2018 International Conference.
One year after treatment, almost three times more patients treated with Zephyr Valves achieved the target improvement in lung function (≥15% increase in FEV1) compared to patients on medical management alone. In addition, Zephyr patients were able to do more daily activities, such as walking, doing chores and getting washed, with less shortness of breath than patients on medical management alone.
Eligible patients were assessed for collateral ventilation using the Chartis Pulmonary Assessment System. Chartis is a diagnostic that assesses whether or not the technology is effective for the patient.
The company has also developed a companion tool to Chartis called StratX. The company said StratX is a cloud-based quantitative CT analysis service that supports Zephyr valve patient selection and treatment targeting by providing clinically-validated information on emphysema destruction, fissure completeness, and volume. Rose said StratX is a machine learning application.
“Every scan we feed [StratX] makes it a little bit smarter,” Rose said. “It’s one of these deep learning systems that basically uses pattern recognition.”
He added, “the other trend StratX fits into is personalized treatment or precision medicine. What you’re seeing [with Zephyr] is the device industry stepping up and bringing a truly tailored precision medicine approach to COPD.”
Zephyr was originally developed by Emphasys, which received the CE mark in 2003. Emphasys went bankrupt in 2009. Pulmonx's investors raised enough money to buy Emphasys's assets.
About the Author(s)
You May Also Like




[42].jpg?width=300&auto=webp&quality=80&disable=upscale)