St. Jude Medical has some compelling clinical and real-world data that it will use to gain better reimbursement coverage for its implantable heart-failure treatment device.
February 12, 2016

St. Jude Medical has some compelling clinical and real-world data that it will use to gain better reimbursement coverage for its implantable heart-failure treatment device.
Arundhati Parmar
St. Jude Medical has had a tough go of getting reimbursement coverage for the pricey product that is CardioMEMS, the company's novel implantable heart failure management device.
In its most recent quarterly earnings conference call, St. Jude Medical simply annualized what the Minnesota device maker garnered in CardioMEMs revenue in the fourth quarter - $16 million to forecast what it will get by selling it in all of 2016 - $65 million. That's even lower than the $70 - $90 million the company had forecast for product sales in 2015.
It has been a disappointment to say the least for analysts who, by the company's own guidance had been modeling for much more.
But in the company's annual investor meeting on Feb. 5 St. Jude executives made it clear that they weren't giving up on the device without a fight.
"We have a clear pathway for reimbursement for CardioMEMS in 2016," declared Mike Rousseau, the company's new president and CEO, according to an archived webcast of the meeting.
The graphic below shows why St. Jude believes the device, which comprises of an implantable sensor which measures pulmonary artery (PA) pressure, and a transmitter and communication system, is worth fighting for.
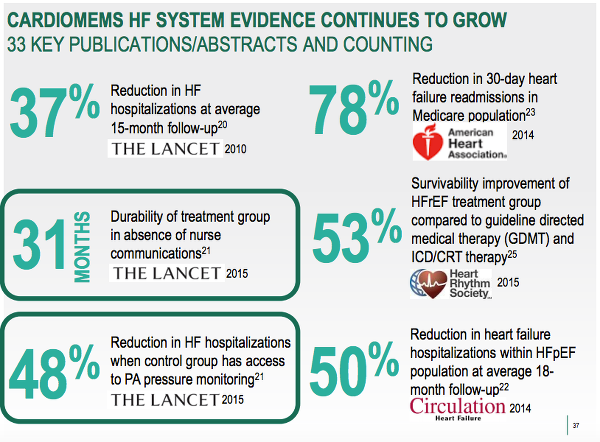
The above shows clinical trials data has proved that the device has significantly reduced heart faliure-related hospitalizations and 30-day readmissions that are expensive to the healthcare system and punitive to hospitals with repeat heart-failure readmissions. The most encouraging data shows 48% reduction in heart failure hospitalizations at 31 months after implantation of the device, showing that the therapy can be sustained.
The 53% figure refers to the improvement in survivability of heart failure patients with reduced ejection fractions and 50% figure refers to reduction in hospitalizations for heart failure patients with preserved ejection fractions. Ejection fraction is a measurement of how well a person's heart is pumping out blood and in diagnosing and tracking heart failure, according to the American Heart Association.
Taking all this data as well as how reduced heart faillure hospitalizations and readmissions can cut healthcare costs when treating heart failure, St. Jude is embarking on a Medicare persuasion campaign. One data point it will use: Less than 25% of hospitals subject to the Hospital Readmissions Reduction Program performed well enough on the CMS' 30-day readmission program to face no penalty.
St. Jude formally submitted a National Coverage Determination application with the Centers for Medicare and Medicaid Services on Jan. 29 and a proposed decision is expected within six months of when the agency decides to accept it. A final decision is not likely before the end of the year.
Meanwhile, St. Jude Medical has convinced a local Medicare Administrative Contractor to reconsider its decision to not cover the device and is working with another MAC that hasn't yet published a final decision.
Both of them will make a call on whether to cover CardioMEMS by the end of the first quarter.
"As you know, reimbursement headwinds are not atypical for new technologies," reminded Eric Fain, group president of St. Jude Medical, analysts and investors.
Arundhati Parmar is senior editor at MD+DI. Reach her at [email protected] and on Twitter @aparmarbb
[Photo Credit: St. Jude Medical]
Get inspired to innovate during Massachusetts Medtech Week—register for BIOMEDevice Boston 2016, April 13-14. |
Correction: An earlier version of the story had an incomplete quote.
You May Also Like


