Netting an Opportunity in Stenting
InspireMD is building a stent product portfolio around its MicroNet technology, aimed at preventing embolization.
November 11, 2016
InspireMD is building a stent product portfolio around its MicroNet technology, aimed at preventing embolization.
A stent is a stent is a stent, right? In what many view as a commoditized market, InspireMD believes it has options for coronary and carotid stenting that could give patients an added layer of protection.
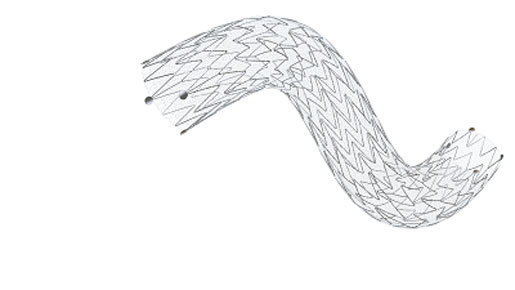
The company has developed its MicroNet technology for embolic protection during coronary and carotid artery stenting. MicroNet is a thin, 20-micron polyethylene terephthalate mesh that is designed to trap plaque against the arterial wall instead of allowing it to flow through the stent. InspireMD has incorporated MicroNet technology into its MGuard and MGuard Prime Embolic Protection Systems as well as the CGuard Embolic Prevention System (EPS). MGuard and MGuard Prime are intended to provide embolic protection during use in percutaneous coronary intervention (PCI) procedures for acute ST-segment elevation myocardial infarction (STEMI) or non-STEMI patients. CGuard, for the carotid artery, is intended to address peri-procedural and late embolization. All three products have CE Mark, but are not approved in the United States.
James Barry, PhD, president and CEO of InspireMD, told MD+DI that the company's products exhibit an "elegant simplicity" and address medical issues that come with "very devastating consequences."
"In the case of MGuard Prime, we're talking MIs (myocardial infarctions) where embolization can lead to death but also . . . you just can't treat it. Even if a patient survives it, their quality of life is terrible thereafter because they usually go into heart failure. Stroke in the brain--very devastating. Both of those have tremendous impact on the healthcare system as well," Barry said.
Learn "How to Set Your Connected Health Solution Apart" at BIOMEDevice San Jose, December 7-8. |
In early November, 12-month data on use of the CGuard EPS in 101 consecutive patients with carotid artery stenosis was presented at the Transcatheter Cardiovascular Therapeutics (TCT) conference by Piotr Musialek, MD, DPhil, FESC from the Jagiellonian University Department of Cardiac and Vascular Diseases in Krakow, Poland. Findings from the PARADIGM-101 study showed 99.1% device and procedure success, no device-related adverse events at 12 months, and vessel narrowing that was reduced from 83+9% to 6.7+5% (p<0.001). There was one peri-procedural minor stroke by 30 days, but no peri-procedural deaths, major strokes, or myocardial infarctions. Healing and patency of the carotid artery was normal at 12 months, Musialek reported.
Musialek pointed out during the presentation that 55% of the patients were symptomatic, but that the findings were important for asymptomatic patients with carotid artery diseases too. "These patients are frequently denied intervention due to fear of the complications associated with conventional intervention, and are left with a substantial risk of stroke when treated only with medication," he said in a press release. "Our study found that CGuard EPS is applicable in up to 90% of all-comer patients with carotid stenosis. These data indicate that CGuard EPS may fundamentally alter the paradigm for managing patients with carotid artery disease, whether they have symptoms or not."
The findings demonstrated sustainability of benefit and the lack of any adverse events through 12 months, Barry added.
Despite good data, the company's products are not yet enjoying widespread adoption. Barry said that although OUS sales commercial performance is "not as good as we would like," MGuard does enjoy "very loyal users." InspireMD's products are delivered in the same way as a conventional stent, so physicians don't need to learn new techniques. However, the coronary stent system is at a disadvantage because it doesn't have a drug on it, he said.
"Drug-eluting stents are sort of carrying the day today and that's been the issue with MGuard. We're still very interested in looking for a partner that has a good commercially available drug-eluting stent," Barry said.
CGuard has been growing commercially, Barry said, with good performance in Israel and Italy, markets where it was introduced first.
"I think it's a matter of just getting the markets up and getting a little bit more visibility," Barry said.
InspireMD is also working toward FDA approval for CGuard. Barry explained that the company plans to approach FDA and would ideally be able to file for an IDE in 2017. Speaking with MD+DI after the TCT data presentation, he said, "There were a lot of our U.S. advisors sitting in that room and they've sort of been beating on us because they would love to get this into the U.S."
Carotid artery stenting has been gaining favor among medical societies and there are carotid artery stents that have passed FDA regulatory scrutiny. However, reimbursement is a barrier. Though CMS does cover carotid artery stenting in specific patients, the procedure is not yet broadly reimbursed compared to the surgical approach of carotid endarterectomy.
One positive development is CMS's September decision to cover TransCarotid Artery Revascularization (TCAR) procedures that use an FDA-approved proximal embolic protection device and FDA-approved carotid artery stent system with a transcarotid indication--Silk Road Medical, the company that made the CMS announcement, offers both types of devices. Those procedures have to be added to a national TCAR Surveillance Project in order to secure reimbursement.
"The fact that [Silk Road has] reimbursement in the U.S. leads you to believe that stents are not going to be far behind," Barry said. "We would obviously like to be part of that."
While expanding use of CGuard is the company's near-term focus, there is plenty of room for additional applications, such as peripheral and neurovascular devices. According to an investor presentation, the company is developing NGuard, a neurovascular flow diverter, and PVGuard, for use in the legs.
"I think the company has great potential. The MicroNet itself is a good platform," Barry said.
[Image courtesy of INSPIREMD]
About the Author(s)
You May Also Like


