Good Reimbursement News for Boston Scientific's Watchman
Medicare's final decision memo on reimbursement coverage for percutaneous left atrial appendage closure therapy is more forgiving than its November proposal. Here's what that means for Boston Scientific's Watchman device.
February 9, 2016
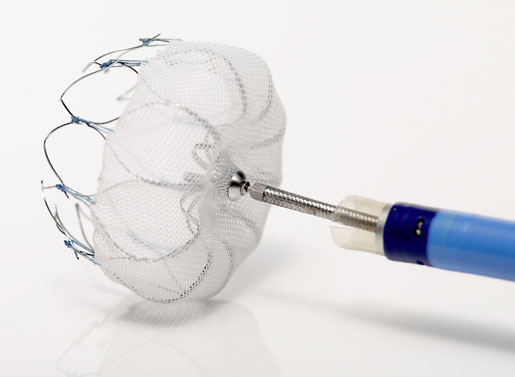
Medicare's final decision memo on reimbursement coverage for percutaneous left atrial appendage closure therapy is more forgiving than its November proposal. Here's what that means for Boston Scientific's Watchman device.
Marie Thibault
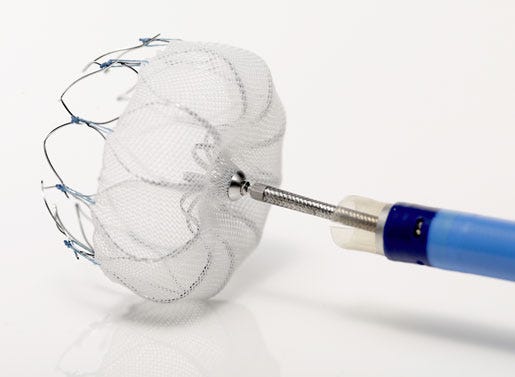
It's a win for Boston Scientific's Watchman device.
CMS released its decision memo on percutaneous left atrial appendage closure (LAAC) therapy on Monday with revised criteria for reimbursement coverage. Notably, the troublesome requirement that patients have a contraindication to warfarin was replaced with a requirement that patients be suitable for short-term use of warfarin but not able to take oral anticoagulants long term.
This change more closely aligns Medicare's coverage requirements with FDA's approval order in March 2015 that noted devices should be used in patients "suitable for warfarin."
CMS acknowledged the change, writing that "our intent was to cover the population within the FDA labeled indication. We have modified our decision to clarify this point . . . Additionally, we consulted with the FDA to ensure that our decision is aligned with the FDA labeled indication."
This determination of patient short-term warfarin suitability with the inability for long-term oral anticoagulant therapy should be the result of "shared decision making," according to the final memo.
During the comment period for the November proposal, clinicians and medical societies showed strong support for Watchman. In December, the leaders of the Society for Cardiovascular Angiography and Interventions (SCAI), the Heart Rhythm Society (HRS), and the American College of Cardiology (ACC) wrote a comment letter to CMS with recommendation for revisions.
CMS appears to have taken those recommendations seriously. The final decision memo reflects key changes suggested in comment letters, including the removal of a requirement to include a registry control arm of contemporaneous patients on oral anticoagulants, as well as a more specific definition of a high stroke risk score. Patients should now have a CHADS2 score of 2 or higher or a CHA2DS2-VASc score of 3 or higher, but mention of a high HAS-BLED bleeding risk score was removed. CMS wrote that "bleeding risk assessment was not a pre-specified outcome parameter in the FDA PMA trials. Further, bleeding risk is often viewed as a contraindication and therefore outside of the FDA label. Therefore, we removed this requirement."
In a February 8 research note, Wells Fargo senior analyst Larry Biegelsen wrote that the specificity on the stroke risk score does make CMS's coverage "slightly more restrictive than the FDA label . . . however, we do not expect this to impact adoption." He pointed out that in the PREVAIL study, one of the trials used to support FDA approval of Watchman, patients were required to have a CHASD2 score of 2 or higher.
Boston Scientific cheered the news Monday in an email statement from a company spokesperson:
Boston Scientific is very pleased with CMS’s national coverage determination (NCD) to cover percutaneous LAAC therapy for select Medicare beneficiaries deemed appropriate by the FDA label. This decision provides consistent, appropriate coverage for the WATCHMAN Device, and takes into account more than a decade of clinical trial data and strong support from physicians and ACC, HRS, and SCAI during the 30-day public comment period."
Other changes included the addition of cardiovascular surgeons as one of the allowed types of implanting physicians and the requirement that the provider involved in "a formal shared decision making interaction . . . on oral anticoagulation" be an "independent non-interventional physician."
Last week, Boston Scientific management were upbeat on Watchman's global market potential, citing at least a $500 million global market opportunity, despite reimbursement uncertainty as it waited for Medicare's release. Analyst Biegelsen wrote in his note that he forecasts $400 million in Watchman sales in 2020 and that the decision "increases our confidence that BSX can achieve our estimates."
Get inspired to innovate during Massachusetts Medtech Week—register for BIOMEDevice Boston 2016, April 13-14. |
Marie Thibault is the associate editor at MD+DI. Reach her at [email protected] and on Twitter @medtechmarie.
[Image courtesy of BOSTON SCIENTIFIC CORP.]
About the Author(s)
You May Also Like


