In Future, TAVR Benefit May Come to Lower Risk Patients
Edwards Lifesciences is embarking upon a clinical trial in the U.S. to test its latest transcatheter heart valve on younger patients with severe aortic stenosis.
January 15, 2016
Edwards Lifesciences is embarking upon a clinical trial in the U.S. to test its latest transcatheter heart valve on younger patients with severe aortic stenosis.
Arundhati Parmar
So far, the benefit of transcatheter aortic valve replacement therapies have come to be felt exclusively by patients deemed too sick to bear open-heart surgery or who have a high-risk of undergoing that invasive procedure.
But now there are efforts to broaden the therapy's availability to even lower risk patients who suffer from aortic stenosis where the heart's aortic valve becomes narrowed. That can lead to breathlessness, chest pain or tightness and decline in activity levels, among other symptoms.
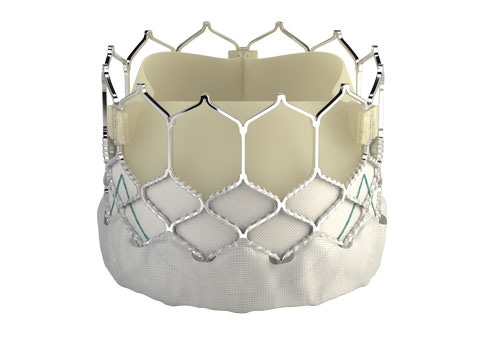
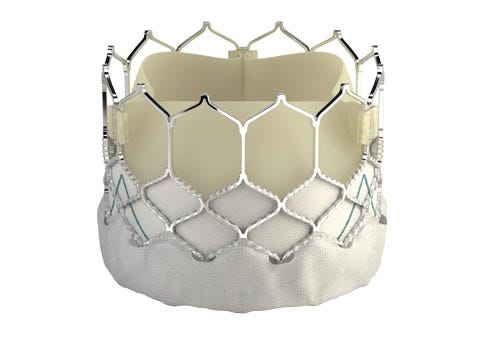
Edwards Lifesciences, the Irvine, California heart valve maker, announced Friday that FDA has approved a study for an expanded indication of its latest transcatheter aortic heart valve, the Sapien 3.
The study will enroll elderly patients with severe aortic stenosis who cardiology experts have determined to be at low risk for mortality if they were to undergo surgical aortic valve replacement. The Partner III trial will enroll up to 1,300 patients at up to 50 sites beginning in the second quarter. Patients must be at least 65 years old and determined by a heart team to have a surgical risk score of less than 4% according to the Society of Thoracic Surgeons adult cardiac surgery risk calculator.
The trial will also include a 400-patient sub-study using advanced imaging to evaluate leaflet motion in tissue heart valves. That is the direct result of the revelation that there is reduced valve leaflet mobility in TAVR patients no matter what kind of heart valve is used as well as in surgical valve patients. One Wall Street analyst called that anomaly a "class effect" noting that there was no statistically significant difference between the valve types and there were no negative outcomes related to this anomaly. But it's something that needs to be watched, concluded heart specialists at the Transcatheter Cardiovascular Therapies Conference in San Franscisco in October.
Meanwhile, like Edwards Lifesciences, other companies are eyeing the possibility that one day TAVR will be approved for patients who are at lower risk of undergoing open-heart surgery. One such company is JenaValve Technology. The Irvine, California firm has a first-generation TAVR valve approved in Europe but is embarking on a path to clinically test a next-generation heart valve that executives believe will be able to rival, and maybe even best, products from established players.
Arundhati Parmar is senior editor at MD+DI. Reach her at [email protected] and on Twitter @aparmarbb
You May Also Like


