FDA Files Consent Decree Against Medtronic and its CEO
FDA files a consent decree against Medtronic, its CEO and Neuromodulation unit president for repeatedly failing to correct violations related to manufacturing of the SynchroMed II Infusion Pump.
April 28, 2015
Arundhati Parmar
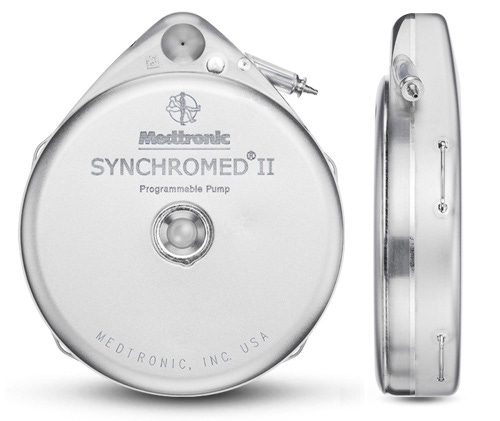
Medtronic's SynchroMed II Implantable Infusion Pump |
A new report from Lifescience Alley, a Minnesota life sciences association, describes how Medtronic, Boston Scientific and St. Jude Medical account for 91% of worldwide neuromodulation sales.
And yet it is this neuromodulation unit of Medtronic that has landed the Irish medtech company in hot water with the FDA. On Monday, the federal agency announced that it has filed a consent decree against Medtronic, it's CEO Omar Ishrak, and the president of the neuromodulation unit, Thomas Tefft, for repeatedly failing to correct violations.
The agency charges that the violations relate to the manufacturing of the SynchroMed II Implantable Infusion Pump Systems that deliver drugs via a programmable implantable device to treat primary or metastatic cancer, chronic pain and severe spasticity. The devices are manufactured at the company's neuromodulation facilities located in Columbia Heights, Minnesota.
Until the matter is resolved, Medtronic cannot legally manufacture and sell the new SynchroMed II Implantable Infusion Pump Systems unless a physician determines that it is medically necessary for a patient, the FDA announcement states.
The agency first approved the Synchromed II Implantable Infusion Pump Systems in 2004. Two years later FDA first identified problems with how they were being manufactured. Such problems can lead to over- or under-infusion, or a delay in patient therapy.
Between 2006 and 2013, FDA conducted five inspections that generated three warning letters, according to the FDA news release. The violations include "inadequate processes for identifying, investigating, and correcting quality problems with the Synchromed II Implantable Infusion Pump Systems; failure to document design changes; and failure to ensure that finished products meet design specifications" FDA notes.
"Medtronic has been working under a Warning Letter for some time now in Neuromodulation (issued August 2012), and it seems that things were just taking too long for the FDA," wrote Joanne Wuensch, an analyst with BMO Capital Markets in a research note on Monday.
The company will be required to hire an independent expert who can help to develop and submit plans for correcting the problems. Wuensch noted that Medtronic has already agreed with FDA on design changes to the SynchroMed device, improvements to its quality system, and ways to keep the device available to physicians should they deem it necessary.
For instance, if patients need a replacement drug infusion pump, Medtronic will need to collect a physician certification before providing a SynchroMed drug infusion pump. The company is required to inform both the physician and the patient about the consent decree with FDA, explained Cindy Resman, a Medtronic spokeswoman, in an email. For new patients, Medtronic has to collect documents from the physician showing that the SynchroMed drug infusion pump is medically necessary.
"Efforts are well underway to address issues included in the consent decree and we will continue our work to implement design changes to the SynchroMed drug infusion pump and enhance the Neuromodulation quality system," Resman wrote. "We are committed to working collaboratively with the FDA to address their expectations in a timely and thorough manner."
The SynchroMed device accounts for less than 1% of Medtronic's overall sales, according to Wuensch, the analyst with BMO Capital Markets. In fiscal 2015, which will end on April 30, sales of the device are estimated to be $306 million worldwide with $204 million expected to come from U.S. sales.
Arundhati Parmar is senior editor at MD+DI. Reach her at [email protected] and on Twitter @aparmarbb
Stay abreast of industry trends at BioMEDevice Boston, May 6-7 at the Boston Convention & Exhibition Center |
You May Also Like


