This Is How St. Jude Is Taking on Medtronic's Neurostim Business
June 15, 2015
FDA approval opens the door for St. Jude Medical's Brio to compete with Medtronic's Activa in the United States.
Chris Newmarker
|
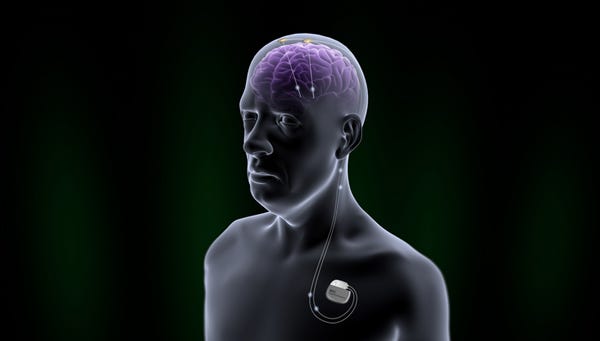
Illustration of the Brio neurostimulation system (Image courtesy of St. Jude Medical) |
Medtronic is no longer the only option in the U.S. when it comes to using an implantable neurostimulator to treat Parkinson's disease.
FDA last week announced approval of St. Jude Medical's Brio neurostimulation system to help reduce the symptoms of Parkinson's disease and essential tremor.
About 1 million U.S. residents have Parkinson's, with about 50,000 new cases diagnosed annually, according to the National Institutes of Health.
"There are no cures for Parkinson's disease or essential tremor, but finding better ways to manage symptoms is essential for patients," William Maisel, MD, acting director of the FDA's Office of Device Evaluation, said in a news release. "This new device adds to the array of treatment options to help people living with Parkinson's and essential tremor enjoy better, more productive lives."
The Brio is slightly smaller than Medtronic's Activa. The Brio is 1.9-by-2.1-by-0.4 in., while the Activa PC is 1.9-by-2.6-by-0.6 in.
The overall concept is pretty similar, however. As with the Activa, the Brio is a pacemaker-like electrical generator implanted under the skin of the upper chest, with leads running to electrodes inside the brain for targetted stimulation.
FDA reports that two clinical studies, one involving 136 Parkinson's patients and another involving 127 essential tremor patients, showed statistically significant improvement when the device was turned on. The majority of patients with essential tremor who used the device were even able to control their symptoms without the need for medications.
The Brio is just the second device approved in the U.S. for treatment of Parkinson's and essential tremor. Medtronic's Activa deep brain stimulation therapy system was approved in 1997 for treating tremor associated with essential tremor and Parkinson's disease, with indications for Activa expanded in 2002 to include the symptoms of Parkinson's disease.
Medtronic in recent years has been working to transform Activa into not only a Parkinson's treatment device, but also a brain activity information recorder that could help researchers gain new insights into the disease.
Refresh your medical device industry knowledge at MEDevice San Diego, September 1-2, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like