How 23andMe Made Its Comeback
November 12, 2015
Nearly two years after the genetic testing company drew FDA's wrath, it is back selling health-related tests.
Chris Newmarker
|
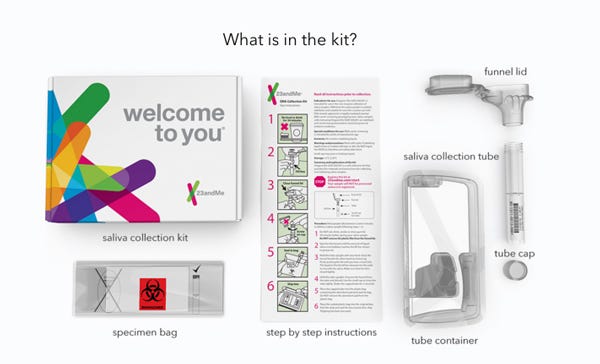
The contents of 23andMe's saliva collection kit. (Image courtesy of 23andMe) |
Blood testing Theranos may be the latest Silicon Valley company to seek to disrupt healthcare, only to run into problems with FDA.
But the story of genetic testing company 23andMe's return to health-related tests demonstrates that the damage does not have to be permanent.
Theranos' regulatory troubles could cost them over $1 million, according to one regulatory expert. And that doesn't even count potentially lost business opportunities amid a series of critical pieces about Theranos' technology in The Wall Street Journal.
It is worth noting, though, how troubled things looked for 23andMe in late 2013 when it selling health-related genetic test analysis after an especially scathing FDA warning letter that accused it of marketing its tests to provide "'health reports on 254 diseases and conditions,' including categories such as 'carrier status,' 'health risks,' and 'drug response,' and specifically as a 'first step in prevention' that enables users to 'take steps toward mitigating serious diseases' such as diabetes, coronary heart disease, and breast cancer."
Since then, the company's founder and CEO Anne Wojcicki made resolution with FDA a priority.
"It was just a question of saying, 'You know what? We entirely need to refocus. We need to hire the right people. We had a major miscommunication. We need to make sure we're executing the right way," Wojcicki recently told BloombergBusiness.
Last month--after two years of cooperation with the FDA, user comprehension testing, and a complete redesign--23andMe was able to launch an entirely new $199 testing experience that includes carrier status, wellness, trait and ancestry reports.
Note that the carrier status tests are not intended to diagnose a disease, or tell someone about their risk for developing a disease in the future. The value is instead to help someone determine whether they are carrying genes for such diseases as cystic fibrosis and sickle cell anemia that might be passed on to future children.
Investors apparently see value in the tests; 23andMe in October raised $115 million in a Series E round led by Fidelity Management & Research Co.
As for Wojcicki, she tells Bloomberg that she's wedded to the company for the rest of her life. "So I'm not interested in selling."
Learn more about cutting-edge medical devices at BIOMEDevice San Jose, December 2-3. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like