HeartWare Faces Problems with Its MVAD
October 13, 2015
A clinical trial of HeartWare's newest LVAD has been paused, at the same time that St. Jude Medical's HeartMate 3 has won CE Mark.
|
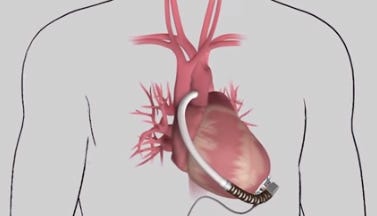
HeartWare's MVAD is one-third the size of its HVAD pump. (Image courtesy of HeartWare) |
Nancy Crotti
HeartWare International has put a clinical trial of its new MVAD Ventricular Assist System on hold--a move that caused investors to head for exits this week.
The Framingham, MA-based company's stock dropped by 27% from Monday morning to noon Tuesday, to $36.70.
In an SEC filing, the company said it had "paused" a trial seeking the CE Mark on September 9 to address an MVAD controller manufacturing process issue. "HeartWare may not re-initiate enrollment in the MVAD clinical trial in November as it previously expected," it continued.
The news for HeartWare and its investors comes on the heels of its major competitor, St. Jude Medical, winning the CE Mark for the HeartMate 3 Left Ventricular Assist SystemDevice (LVAD). St. Jude acquired the HeartMate 3 through its $3.3 billion purchase of Thoratec last week.
The HeartMate 3 could address some of the problems U.S. FDA has noticed with LVADs. Since FDA approval of the devices, there has been an increased rate of pump thrombosis (blood clots inside the pump) among people using Thoratec's HeartMate II, and a high rate of stroke among people using the HeartWare HVAD, FDA says.
FDA officials still maintain that the benefits of LVADs outweigh the risks when it comes to saving the lives of people with advanced left ventricular heart failure.
In the case of HeartWare and its MVAD, the company says it began investigating causes of reported adverse events in certain clinical trial patients, following discussions with its trial investigators. "The events being analyzed are typical of those seen in other clinical trials for ventricular assist devices," HeartWare continued in an SEC filing. "HeartWare took similar actions successfully during its initial human study for the HVAD System during the HVAD CE Mark clinical trial in 2007."
The company advised investors to stay tuned for its third-quarter financial report and conference call scheduled for October 29. HeartWare said it remains confident in its MVAD system and its potential to help patients. The company said it intends to present and publish clinical data following the completion of enrollment and follow-up for all trial patients.
The MVAD is one-third the size of HeartWare's HVAD Pump. During an analyst call last month, HeartWare CEO Doug Godshall said 11 patients had enrolled in the clinical trial.
The company found that when it shifted from engineers to operators building the controllers, the operators were putting too much stress on one of the device's circuit boards, Godshall said in a transcript of the call provided to the SEC.
"We found a way to reduce the stress, but we have to add some fixtures and some other work to make sure that the yields improve, and we have certainty of quality of the controller at the end of the line," he added.
Learn more about cutting-edge medical devices at Minnesota Medtech Week, November 4-5 in Minneapolis. |
Nancy Crotti is a contributor to Qmed and MPMN.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like