Guess Which State Has Won the Most PMAs
March 2, 2015
California may be the largest medtech state, but it is not at the top of the list when it comes to U.S. FDA approvals for the toughest type of medical device applications.
Chris Newmarker
It turns out that when it comes to getting premarket approvals from FDA, Minnesota remains the leader and has even grown its lead over California during the Great Recession, according to EvaluateMedTech data relayed by LifeScience Alley.
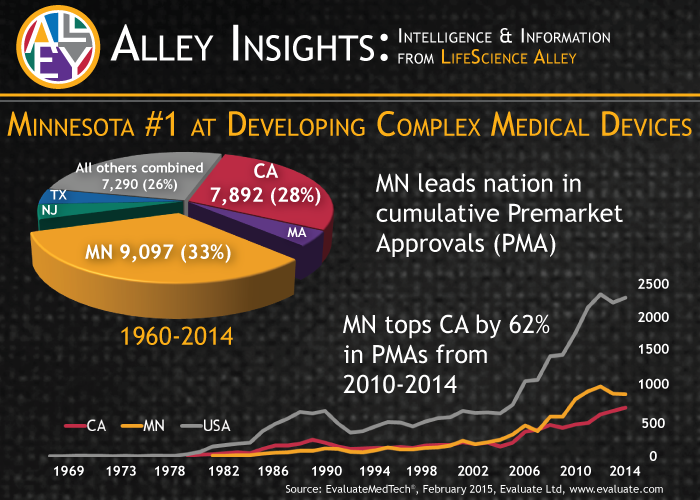
Over more than 50 years of PMAs, a third of them--more than 9000--involved devices created by Minnesota applicants. California only had 28%, or nearly 8000. And LifeScience Alley reports the difference has been even more stark in recent years.
|
Minnesota trumps California when it comes to the volume of PMAs. Graphic from Life Science Alley. |
The next states in the running--Massachusetts, Texas, and New Jersey--only had a fraction of the pie.
Minnesota's accomplishment is even more remarkable considering that California easily wins out when it comes to number of medtech workers (about 63,207 to Minnesota's 28,141, as of 2013) and venture capital funding ($1.01 billion to Minnesota's $165 million, as of 2013).
California is home to more medical device companies than any other U.S. state, with a dense network of high-tech firms and a talented workforce. But some of the most life-saving (as well as most highly regulated) devices have been created in Minnesota.
That makes sense considering the major medtech companies that are either based in Minnesota or have major operations there, including Medtronic, St. Jude Medical, and Boston Scientific. Think pacemakers, defibrillators, and neuromodulation devices.
Find out more about efforts to preserve Minnesota as a Midwest medtech hub.
Refresh your medical device industry knowledge at BIOMEDevice Boston, May 6-7, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like