On-Body Drug Delivery System Reduces User Error & Waste
Vertiva’s reusable controller and prefilled, preloaded cartridges deliver precise basal and bolus injections for multiple therapies.
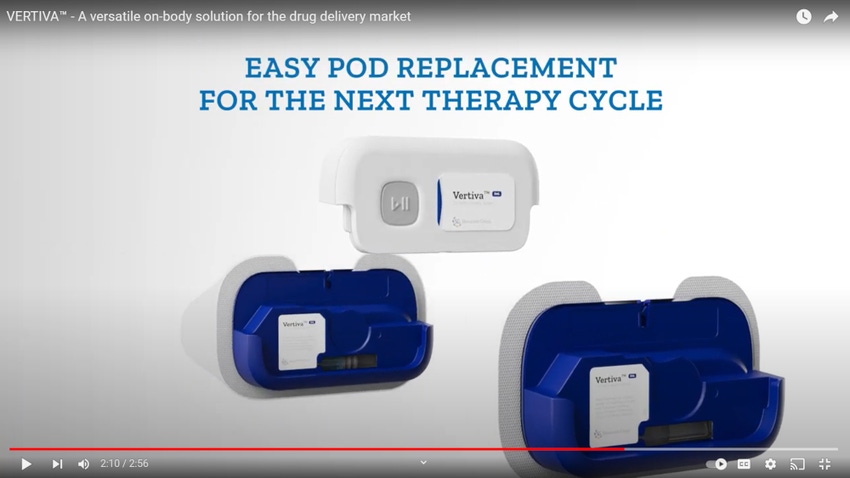
Stevanato Group S.p.A., in collaboration with Thermo Fisher Scientific, has launched Vertiva, a semi-reusable drug-delivery device for subcutaneous administration of injectable therapies. The on-body delivery system allows for small molecules and biologics to be delivered at home, improving patient adherence and treatment success.
The device comprises a multi-use container and a single-use pod containing a cartridge. Stevanato Group supplies the device platform, pre-sterilized EZ-fill cartridges for use as drug containment solutions, and the assembly equipment. Thermo Fisher provides fill-and-finish and final assembly services.
Stevanato Group
“Vertiva works with standard ISO glass cartridges and large volumes from 3 to 10 milliliters,” says Riccardo Butta, president, Americas at Stevanato Group. “Customers can choose Stevanato Group’s bulk or EZ-fill glass cartridges, which are already optimized for integrating with Vertiva, or ISO glass cartridges produced by other companies.”
Butta explains that Vertiva is offered to pharmaceutical customers as an integrated device and as a fill-and-finish solution — thanks to a strategic collaboration with Thermo Fisher Scientific — to streamline management of the pharmaceutical supply chain. The cartridges containing the drugs are preloaded and sealed into the pod during factory assembly. Production and subassembly of Vertiva take place at Stevanato Group’s device manufacturing site in Germany. Final assembly and packaging occur at the customer location or its selected contract manufacturing organization (CMO), which could be Thermo Fisher if the customer chooses Stevanato’s CMO service.
Vertiva is also a step forward for Stevanato Group’s sustainability efforts: The device’s reusable controller extends the product’s lifespan and reduces waste, while the disposable pod with the prefilled, preloaded cartridge helps to reduce user error and resultant product waste.
According to Butta, customers received access to Vertiva’s first samples in 2023. The company already has an ongoing collaboration with Bexson Biomedical to treat a range of mental health conditions with another of its wearable delivery systems; the SG EZ-be Pod.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
