Why the 'Turnaround Test' Matters for Medical Devices and Wearables
February 3, 2015
|
Above is Misfit's Bolt smart light bulb. Below is the Swarovski Shine fitness tracker. |
Misfit, the Burlingame, CA-based maker of the Shine and a lineup of other innovative products, is making waves in both the wearables and smart home markets. The origins of the company's philosophy, however, lie in the medical device space--and how company officials cracked what they call the "turnaround test."
Before we get into that, however, let's take a look at what Misfit has accomplished since it was founded in 2011 by former Apple CEO John Sculley and the cofounders of diabetes firm AgaMatrix (Salem, NH), Sridhar Iyengar and Sonny Vu. The company debuted as a wearables start-up and has attracted $63 million in funding and raised nearly $850,000 on the crowdfunding site Indiegogo.
Recently, the company has broadened its focus to bridge the gap between wearables and the smart home. Its latest fitness trackers can also be used to unlock doors, control the thermostat, and even play music via Spotify. The company is also introducing the Bolt, a wireless smart light bulb that can project dynamic scenes on the wall; it can be controlled with a smartphone, which, in a sense, is a wearable device itself.
In addition, the company continues to receive attention for its health-tracking technology. At CES, it debuted the Swarovski Shine, a piece of jewelry that doubles as a fitness tracker.
The company has also launched the Beddit sleep monitor. Affixed to a mattress, the device monitors a user's sleep patterns, heart rate, snoring, and so forth, while beaming the data to a smartphone.
Once it is set up, the Beddit device monitors sleep without the user having to wear anything--unlike Jawbone's and Fitbit's products, which must be strapped to the wearer's wrist to work.
The Story Behind the iPhone-Compatible Glucose Meter
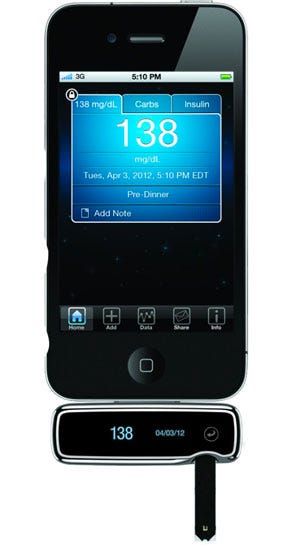
Misfit's approach to product development is inspired by insights won by Agamatrix, a glucose-meter maker founded in 2001. Initially, the company had the aim of using signal processing technology to make glucose meters more accurate. The company's biggest innovation, however, was the iBGStar, a glucose meter that served as an entryway to the mobile health field.
"When the iPhone came out and we realized that it would solve big problems for folks with type-2 diabetes," says Sridhar Iyengar, the cofounder of both Agamatrix and Misfit. Agamatrix realized that the patients with type-2 diabetes were not testing their blood glucose levels frequently enough. "We thought this was because of the pain of pricking their fingers. But it actually turned out that the number one reason people didn't test was they just forgot," Iyengar says.
|
The iBGStar was the first glucose meter that is compatible with a smartphone. |
While patients with type-1 diabetes test and inject themselves everyday, patients with type-2 don't because they either forgot altogether, or they forgot to bring their glucose meter in the morning. On top of that, they oftentimes struggled to interpret the numeric data from the device. "They might think: 'OK, so I have a number. Should I eat salad for dinner or what?'" Iyengar explains.
When the iPhone debuted in 2007, the company was convinced the device could help all of these problems if they could manage to create a glucose meter that connects with the device.
The Turnaround Test
The development of the iBGStar follows from what the company terms the "turnaround test:" If you left home in the morning and later realized you forgot something, would you turn around, go home, and get it?
It turns out that glucose the meter did not pass the turnaround test--especially for type-2 diabetics. "Most people, if they forgot their glucose meter thought, 'Oh, I'll look at it when I get home.' Or they might have a second one in the office," Iyengar says, "But if you forgot your wallet or iPhone, you would go back and get it."
The company then decided to make the iBGStar, a glucose meter that plugs into the iPhone. It worked independently on the iPhone as well, but when connected, the technology made it easy to keep track of trend data, put context around the blood glucose data, and even share data with healthcare professionals, if desired.
The device won considerable acclaim, including the Red Dot Design and the GOOD Design awards.
One thing that surprised the firm, however, was the amount of positive feedback they received from parents OR children with type-2 diabetics--as well as from teens and preteens with the condition. As it turns out, a significant number of teens and preteens were buying the device with their allowance. "This was not a product you could get on insurance. It was a premium product, and you had to pay cash for it," Iyengar says. "That was A) unheard of and B) the parents were writing us in letters saying, 'All of the sudden, my child is testing more, and they are actually interested in monitoring their progress. And the biggest thing was: they are not embarrassed about having diabetes anymore.'" Not only were they not embarrassed but they were also showing off the device to their friends because it looked cool.
But the company hadn't really intended to make a device that turned heads; they only intended to match the Apple design aesthetic. "Yes, we had fun doing it, but we had this huge unexpected reaction regarding the design," Iyengar says. "Nobody cared that the iBGStar was more accurate than competing products. The fact it looked like an Apple product changed their perception and behaviors," he says.
That revelation ultimately became the guiding philosophy for Misfit, which, as cofounder Sonny Vu has explained, seeks to develop products that won't make the user look like a doofus.
But initially, the feedback that Agamatrix received for the Applesque design of the iBGStar was a big revelation. "In hindsight, five or six years later, it is a no brainer. But back then, we thought, 'Wow, people really care about this stuff?'" Iyengar concludes.
Like what you're reading? Subscribe to our daily e-newsletter.Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
About the Author(s)
You May Also Like