Judgment Calls
May 7, 2003
Originally Published MPMNMay 2003
2003 MedicalDesign Excellence Awards
Judgment CallsPart II: The Winners
Critical Care and Emergency Products
Rugged Stair-Pro
The Rugged Stair-Pro manufactured by Stryker EMS (Kalamazoo, MI) is an emergency patient-transport device used to extricate individuals from enclosed or confined locations where a conventional cot would be unable to operate, including stairways. It is designed to cause less strain to users. Design and supply credits go to Scori Mold and
Engineering Inc. (Centerville, MI) and Alexandria Extrusions (Alexandria, MN). 
Angel Telemetry Unit
The Angel telemetry unit manufactured by Medical Data Electronics Inc. (Arleta, CA) is a low-cost, single-patient-use radio transmitter for ambulatory patients. It is designed to provide continuous monitoring of the ECG vital signs of adult and pediatric patients in the hospital environment. 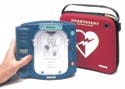
HeartStart Home Defibrillator
The HeartStart home defibrillator manufactured by Philips Medical Systems--Heartstream (Seattle, WA) is the first of a new generation of defibrillators designed specifically for the home. It features a simplified interface and employs technology that senses operator behavior and adapts voice instructions to user actions.
Finished Packaging Easytab Hearing Aid Battery Delivery System
Easytab Hearing Aid Battery Delivery System
The Easytab hearing aid battery delivery system manufactured by Duracell (Bethel, CT) consists of a hearing aid battery featuring a long custom-shaped tab that enables consumers, including those with diminished dexterity, to easily remove the battery from its packaging and insert it into even the smallest hearing aid. Design and supply credits for the Easytab hearing aid battery delivery system go to Product Ventures Ltd. (Fairfield, CT).
General Hospital Devices and Therapeutic Products
SmartSite Plus
The SmartSite Plus needle-free valve with positive displacement manufactured by Alaris Medical Systems Inc. (San Diego, CA) is designed to reduce the incidence of clotting in indwelling catheters and enhance the safety of clinicians and patients by providing passive needle-free access to intravenous systems. Design and supply credits for the SmartSite Plus needle-free valve with positive displacement go to Alaris Medical Systems Inc. (Creedmoor, NC) and Karl Leinsing (Hampton, NH).
Joey Disposable Umbilical Cord Clamp and Cutter 
The Joey disposable umbilical cord clamp and cutter manufactured by Maternus (San Antonio, TX) is a single-use obstetric device that simultaneously clamps and cuts the umbilical cord following birth, while shielding users from blood spray. It is optimized forsingle-handed use, and is color coded for multiple births. Design and supply credits for the Joey disposable umbilical cord clamp and cutter go to Design Edge (Austin, TX) and Trend Technologies llc (Longmont, CO).
Introcan Safety Intravenous Catheter 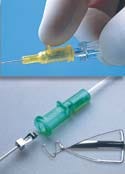
The Introcan Safety intravenous catheter manufactured by B. Braun OEM/Industrial Div., B. Braun Medical Inc. (Bethlehem, PA) is a passive IV catheter that requires no user activation of the safety mechanism. It requires no change in user technique to activate the safety mechanism, and is designed to make conservative use of materials.
Implant and Tissue-Replacement Products
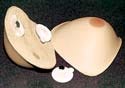 ContourMed Advantage External Breast Prosthesis
ContourMed Advantage External Breast Prosthesis
The ContourMed Advantage external breast prosthesis manufactured by
ContourMed Inc. (Little Rock, AR) is a custom lightweight external breast prosthesis with a magnetic attachment for added wearer confidence. Laser scanning and computer-aided modeling are used to ensure accurate replication and a comfortable fit. Design and supply credits for the ContourMed Advantage external breast prosthesis go to Webtec Converting llc (Knoxville, TN), BJB Enterprises Inc. (Tustin, CA), 3MMedical Specialties (St. Paul, MN), and General Electric Silicones (Atlanta, GA).
Ascension Metacarpalphalangeal Joint Implant 
The Ascension metacarpalphalangeal joint implant manufactured by Ascension Orthopedics Inc. (Austin, TX) is intended as a total replacement for the metacarpalphalangeal joint of the hand. Its design replicates natural structures and reproduces joint kinematics, thereby restoring functions such as pinching and grasping. Design and supply credits for the Ascension metacarpalphalangeal joint implant go to Medical Carbon Research Institute (Austin, TX), Banister Tool Co. (Austin, TX), and Symmetry Medical (Warsaw, IN).
In Vitro Diagnostics
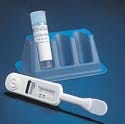
 Accu-Chek Inform Blood Glucose Meter
Accu-Chek Inform Blood Glucose Meter
The Accu-Chek Inform blood glucose meter manufactured by Roche Diagnostics (Indianapolis, IN) is a professional-use blood glucose meter that has been designed for multiple patient testing in hospitals. The device is based on a personal data assistant platform, from which test results can be downloaded. Design and supply credits for the Accu-Chek Inform blood glucose meter go to Kinneir Dufort (Bristol, UK).
OraQuick Rapid HIV-1 Antibody Test
The OraQuick Rapid HIV-1 antibody test manufactured by OraSure Technologies Inc. (Bethlehem, PA) is asingle-use, qualitative immunoassay to detect antibodies to HIV-1 in a fingerstick whole-blood specimen. FDA has granted this test waived status under the Clinical Laboratory Improvement Amendments of 1988. Design and supply credits for the OraQuick Rapid HIV-1 antibody test go to Millipore (Bedford, MA), Inland Technologies Inc. (Fontana, CA), and Innovations (Oakdale, CA).
Over-the-Counter and Self-Care Products
Symphony Breast Pump 
The Symphony breast pump manufactured by Medela Inc. (McHenry, IL) combines the results of the latest human lactation research with microprocessor technology. It features a two-phase pumping program with stimulation and expression cycles to promote the milk-ejection reflex and maximize milk flow. Design and supply credits for the Symphony breast pump go to Milani Design & Consulting SA (Erlenbach, Switzerland).
PiKo-1 Handheld Lung-Function Monitor
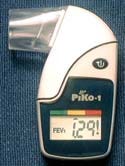
The PiKo-1 handheld lung-function monitor manufactured by Pulmonary Data Services Inc. (Louisville, CO) is a small handheld device for home monitoring of lung-function parameters, including forced expiratoryvolume, and is designed to predict an oncoming asthma attack before it becomes symptomatic.
Radiological and Electromechanical Devices
MammoSite Radiation Therapy System 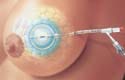
The MammoSite radiation therapy system manufactured by Proxima Therapeutics Inc. (Alpharetta, GA) is a single-use catheter and balloon assembly that delivers radiation from within the tumor-resected cavity after breast conservation therapy. It is designed to shorten total treatment time by up to 85%. Design and supply credits for the MammoSite radiation therapy system go to ETI Inc. (Largo, FL), Proximal Inc. (Largo, FL), and Medron (Salt Lake City, UT).
iLook Personal Imaging Tool 
The iLook personal imaging tool manufactured by SonoSite Inc. (Bothell, WA) is a full-featured, all-digital ultrasound imaging tool that weighs only 3 lb. Its light weight and small size enable physicians and technicians to easily carry ultrasound capabilities into a variety of clinical settings.
Sonos 7500 Live 3-D Ultrasound System
The Sonos 7500 Live 3-D ultrasound system manufactured by Philips Medical Systems (Andover, MA) is a fully functional premium ultrasound system that provides real-time 3-D cardiac imaging.
Rehabilitation and Assistive-Technology Products UroCycler Bladder Management System
UroCycler Bladder Management System
The UroCycler bladder management system manufactured by UroSolutions Inc. (Orlando, FL) is a passive, low-pressure magnetic valve that enables the bladder to fill and void in a physiologically consistent manner, preventing backflow and reducing potential for infection. Design and supply credits for the UroCycler bladder management
system go to Bradshaw Manufacturing Services Inc. (Palm Bay, FL) and International Sterilization Laboratory Inc. (Groveland, FL).
Cozmo Insulin Pump and My Treatment Assistant PC 
The Cozmo insulin pump and My Treatment Assistant PC communications are manufactured by Deltec Inc. (St. Paul, MN). The Cozmo insulin pump is a full-featured, flexible, and easy-to-use device for administering insulin to individuals with diabetes. It features a user-friendly interface and consumer-product design, making it less intimidating for users.
Design and supply credits for the Cozmo insulin pump and My Treatment Assistant PC communications go to Bridge Design (San Francisco, CA) and Advanced Molding Technologies (St. Paul, MN).
Taylor Spatial Frame System
The Taylor Spatial Frame and spatial frame.com orthopedic fixator system manufactured by Smith & Nephew Inc., Orthopaedic Div. (Memphis, TN) is an orthopedic device that encompasses an external fixator frame for aligning bone fragments, and Web-based software for developing a patient's corrective plan and directing daily adjustments to the frame. Design and supply credits for the Taylor Spatial Frame and spatial frame.com orthopedic fixator system go to NetIDEAS Inc. (Mt. Laurel, NJ).
Surgical Equipment, Instruments, and Supplies A-2000 Bispectral Index Consciousness Monitor
A-2000 Bispectral Index Consciousness Monitor
The A-2000 bispectral index (BIS) consciousness monitor manufactured by Aspect Medical Systems (Newton, MA) is a compact, portable unit that translates information from an electroencephalogram to measure a patient's depth of consciousness in any operating room environment, thereby reducing overmedication and related side effects. Design and supply credits for theA-2000 consciousness monitor go to Design Continuum Inc. (West Newton, MA),Tech Design (Merrimack, NH) and William J. Wholtmann (San Jose, CA).
Zeus Robotic Endoscopic Surgical Workstation 
The Zeus robotic endoscopic surgical workstation manufactured by Computer Motion (Goleta, CA) is a robotic computer-enhanced endoscopic surgical workstation for minimally invasive microsurgery procedures, such as endoscopic coronary artery bypass. It enhances surgical precision and is suitable for telesurgery procedures. Design and supply credits for the Zeus robotic endoscopic surgical workstation go toPatton Design (Irvine, CA).
Intralase FS Ophthalmological Laser 
The Intralase FS ophthalmological laser manufactured by Intralase (Irvine, CA) is an ergonomically designed femtosecond ophthalmological laser for making the corneal flap required in refractive keratotomy procedures, providing surgeons a safe and precise all-laser alternative to mechanicalmicrokeratomes. Design and supply credits for the Intralase FS ophthalmological laser go to Patton Design (Irvine, CA).
CORx Integrated Oxygenation System 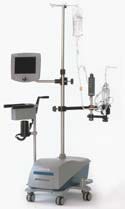
The CORx integrated oxygenation system manufactured by CardioVention (Santa Clara, CA) is a compact disposable driven by a unique hardware platform that integrates automated air removal, blood pumping, and oxygenation functions of the heart and lungs, enabling less-invasive andminimal-hemodilution cardiac surgery. Design and supply redits for the CORx integrated oxygenation system go to Data Spectrum (Redondo Beach, CA), Lunar Design (Palo Alto, CA), damgudesign (Los Angeles, CA), Solid Concepts (Valencia, CA), Emoteq (Tulsa, OK), Volker Precision Products (Sunnyvale, CA), Polymer Technology Corp.(Menomonie, WI), Pacific Plastics & Engineering (Soquel, CA), Plastics Engineerng &Development Inc. (Carlsbad, CA), Delta Pacific (Santa Clara, CA), and Membrana GmbH (Wuppertal, Germany).
Copyright ©2003 Medical Product Manufacturing News
You May Also Like


