Digital Health, Digital Therapeutics, & the Digital Future of Medtech
A single DTx project could lay the groundwork for future-proofing a medical device company’s offerings, says MD&M West speaker Bill Betten.
April 7, 2022
Bill Betten, Director of Solutions, S3 Connected Health
The past two years have seen healthcare embrace contemporary tech to cope with the challenges of the pandemic. For most patients, this was as simple as using commercial video software for a consultation with their doctor; for others it was leaping into the world of remote care devices.
While the level of advancement in digital health in this period is hotly debated, what’s clear is that patients and clinicians are more willing to embrace new tech in healthcare than ever.
Challenges and Opportunities for Medical Device Companies
For medical device companies, this presents a challenge and an opportunity.
How do you keep your device relevant and user-friendly for consumers who are immersed in our digital world of hyper-personalization and convenience? The answer, and the opportunity, is to embrace digital health to update and compliment your existing device offerings.
Among the range of digital health options available, digital therapeutics (DTx) is one newly emerging category that offers significant potential for medical device companies, while promising better outcomes for patients, and the clinical validation to encourage adoption among clinicians and patients.
What Are Digital Therapeutics?
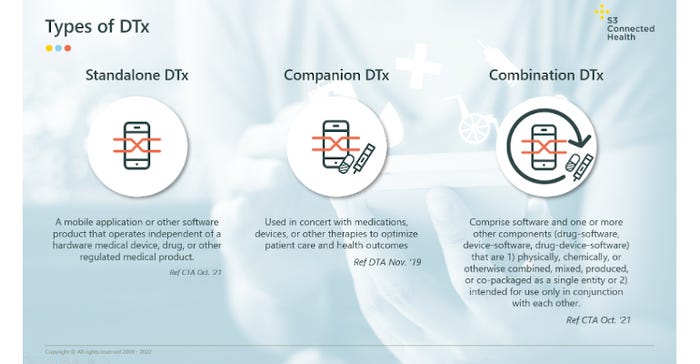
In short, DTx provide software-driven therapeutic interventions for the treatment, management, or prevention of a disease or disorder. They fuse combinations of devices, software, behavioral therapy, data driven interventions, and gamification, extending the value of a device and realizing the potential of captured data to drive better healthcare outcomes. There are three main categories emerging: stand-alone DTx, companion DTx, and combination DTx. See the below infographic for details.
Investment in DTx Is Growing as Regulatory Paths Expand to New Regions
Reuters recently quoted Rock Health figures showing U.S. investment in digital therapeutics products increased 260% between 2020 and 2021. At least 63 approved DTx are now documented in the U.S. open FDA device database, 28 applications have completed the German DiGA process, and other regions such as Australia, Belgium, and South Korea have also introduced regulatory pathways for DTx.
Countries are taking note of the value of DTx, and many can now be prescribed and reimbursed, thanks to their clinically validated nature. DTx are standing apart from the hundreds of thousands of health and wellness apps available for your phone. They provide reassurance to all stakeholders that they do exactly what they claim to and can help DTx/device offerings stand out in an increasingly crowded market.
It's Not Too Late for Medical Device Companies to Embrace Digital Therapeutics
To date most notable DTx have been software-only products, with early pioneers mostly focused on the mental health space, and pharma often concerned with software/drug companions. Only now are medical device companies recognizing the growing opportunity to create DTx that combine a software element with a physical device and/or medication.
These ‘combination’ DTx solutions offer traditional device-based companies the chance to further their capabilities in digital health and provide a more appealing, competitive product while retaining value around their core devices. Even some of the biggest players in the medical device space are getting in on the action, as demonstrated by Philips’s recent joining of the DTA—an organization previously composed of DTx and pharma companies only.
A single DTx project could lay the groundwork for future-proofing a device company’s offering, and the timing is perfect to delve into this promising new area.
Join Us at MD&M West to Learn More about Digital Therapeutics
In this rapidly changing world of connected devices and evolving digital health offerings, what are the implications for traditional medical device developers and providers that don’t embrace newly emerging tech, and what sense can a casual consumer make out of it all?
Please join me at MD&M West in Anaheim on April 12th, when I will discuss the implications of DTx with Gordon Kass of Pear Therapeutics and Bryan Nolan of myBiometry. We will cover:
The relevance of DTx to medtech companies
Obstacles to overcome, and the opportunity DTx represents
Case studies of real-world examples
I hope to see many of you there and answer any questions you have on DTx.
You May Also Like


