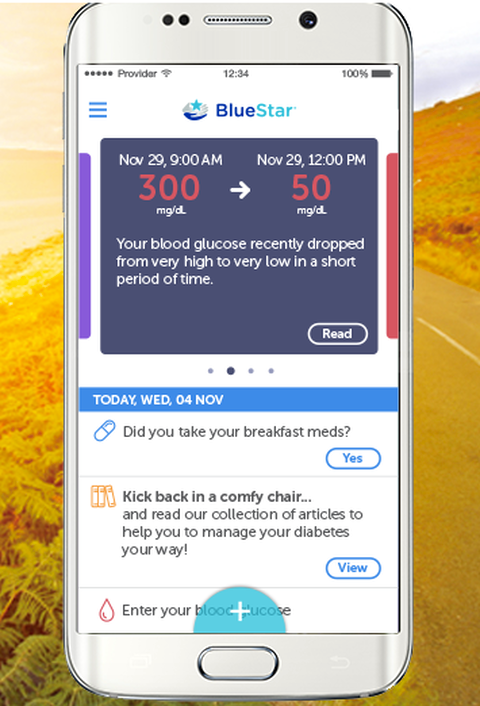
Product Name: BlueStarDescription: BlueStar is the first mobile prescription therapy that is cleared by the FDA to help type 2 diabetes patients manage their disease.
February 22, 2016
Product Name: BlueStar Company: WellDoc Description: BlueStar is the first mobile prescription therapy that is cleared by the FDA to help type 2 diabetes patients manage their disease. The solution is prescribed by physicians, activated by pharmacies and treated like a pharmacy benefit. WellDoc's mobile solutions incorporate, coaching, education and information tailored to individual diabetics based on their behavior and personal data to keep their blood glucose levels controlled and manage the disease.
In 2011, the company proved through a clinical trial that when patients used its diabetes management mobile platform for a 12-month period, they were able to reduce ER visits and hospitalizations by 58%. Further, and more importantly, a clinical trial showed that patients using WellDoc's mobile coaching platform were able to reduce their blood glucose levels more than those who were using traditional methods of disease management. FDA Clearance: 2010
|
You May Also Like