Omnipod 5 Gets Full Market Release in Germany
The Insulet device has also received its medical aid numbers from the German National Association of Statutory Health Insurance Funds, making it reimbursable by health insurers.
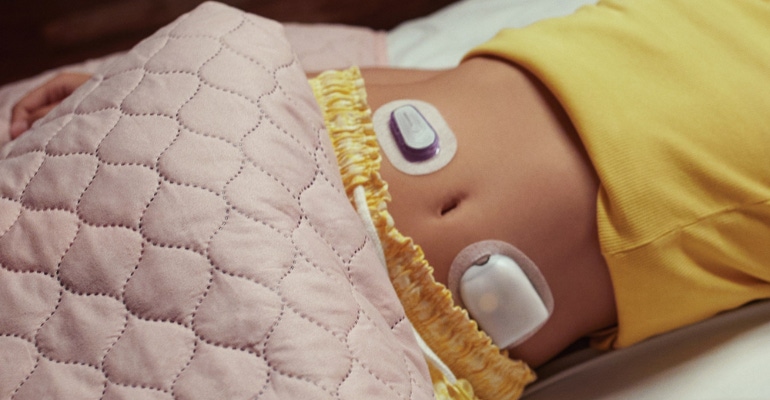
Insulet, a tubeless insulin pump technology company, today announced the commercial launch of its Omnipod 5 Automated Insulin Delivery System in Germany, for people aged two-years and older with type 1 diabetes. The launch marks the company’s inclusion in three separate markets, Germany, the United States, and the United Kingdom.
Omnipod 5 is the first CE-marked tubeless automated insulin delivery system that integrates with the Dexcom G6 Continuous Glucose Monitoring (CGM) system, according to the company. The system “includes the tubeless Pod enhanced with SmartAdjust technology and a handheld controller integrated with the SmartBolus Calculator,” according to the company press release. “The system is interoperable with Dexcom G6 for automated insulin delivery to help protect against high and low glucose levels.”
The insulin pump has seen favorable glycemic outcomes from Insulet pivotal trial real-world data including 36,634 adults and 18,516 adolescents with type 1 diabetes in the US.
In addition to the device now being available in the country, Omnipod 5 has also received its medical aid numbers from the German National Association of Statutory Health Insurance Funds, making it reimbursable by health insurers. Insulet said its goal is to make the device available for the majority of the company’s European customers by the end of 2024.
“We know people in Germany living with type 1 diabetes want the most advanced technology options for making diabetes management easier,” said Trang Ly MBBS, FRACP, PhD, Insulet senior vice president and medical director. “With our impressive data showing improved clinical outcomes from users in the United States over the past year, we are confident in our ability to make a difference in Germany and additional markets in the near future.”
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
