Medtronic Scores MiniMed 780G Win in Canada
The company kicked off Diabetes Awareness Month with Health Canada license for MiniMed 780G.
November 3, 2022
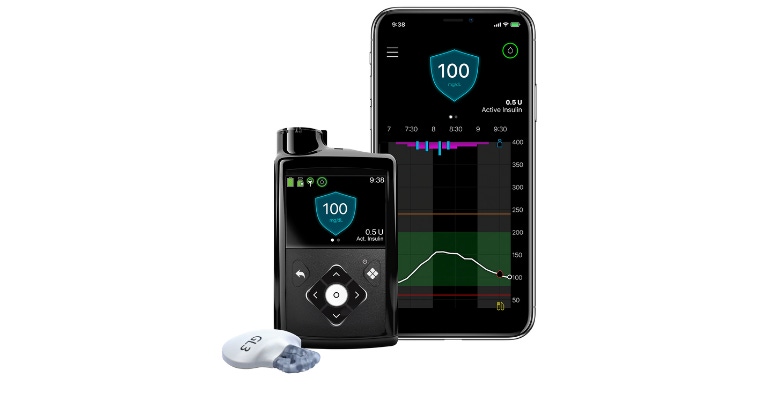
Medtronic kicked off Diabetes Awareness Month with news that its MiniMed 780G system for people living with type 1 diabetes has received a regulatory license from Health Canada. The company touts that the MiniMed 780G is the only insulin pump system in Canada that automatically adjusts and corrects sugars every five minutes — some user interaction is required, however.
The regulatory milestone comes during a year of significant progress in diabetes tech.
The MiniMed 780G system is designed to help avoid highs and lows using Medtronic's SmartGuard technology.
“Our latest system is designed for real life by increasing automation for an easier way to help stabilize sugar levels,” said Laura Cameron, senior director of dndocrinology at Medtronic Canada. “Mealtimes can be stressful, especially when you’re on the go, so we’re pleased to have designed an advanced algorithm that automatically covers for underestimated carbs with meal detection technology."
The SmartGuard technology enables users to personalize glucose goals with an adjustable target setting as low as 5.5 mmol/L and is designed to help stabilize blood sugar levels to further help improve glucose control. This system can also significantly reduce the need for injections — up to 96% for those using insulin pens alone, Medtronic noted.
According to the most recent data from the ADAPT study published in The Lancet Diabetes & Endocrinology, the first multi-national randomized controlled study comparing MiniMed 780G system versus the standard of care (multiple daily injections and continuous glucose monitoring), individuals with type 1 diabetes using the MiniMed 780G system showed significant improvement across all glycemic metrics compared to the standard of care.
The MiniMed 780G is indicated for management of type 1 diabetes in people age 7-80 years, whose total daily dose of insulin is 8 units per day or more. The MiniMed 780G system is intended for the continuous delivery of basal insulin at selectable rates and the administration of insulin boluses at selectable amounts. The system is also intended to continuously monitor glucose values in the fluid under the skin.
About the Author(s)
You May Also Like




