Insulet Issues Voluntary Device Correction for Omnipod 5 Controller
The company has received reports that heat generated by a poor connection between the cable and port has caused them to melt, deform, or discolor.
November 16, 2022

Insulet has received reports that heat generated by a poor connection between the Omnipod 5 cable and port has caused them to melt, deform, or discolor.
Insulet reported a medical device corrections for its Omnipod 5 automated insulin delivery system because of an issue with the unit’s controller charging port and cable. The company said this does not impact its other products, including the Omnipod 5 Pod, the Omnipod Dash insulin management system, the Omnipod insulin management system, or compatible Android smartphone devices that have the Omnipod 5 app installed. These actions are taken voluntarily with the knowledge of FDA.
Insulet has received 24 reports that the Omnipod 5 controller USB-C charging port or USB cable are melting, deforming, or discoloring due to heat generated by a poor connection between the cable and the port. If those areas are touched by the user, the excess heat may cause burns, or the heat could lead to a fire. No serious injuries have been reported to Insulet as a result of this issue.
The company is notifying users of the Omnipod 5 by mail with instructions on how to detect and reduce the risk of an issue with the charging port and cable.
The Omnipod� 5 tubeless automated insulin delivery system is integrated with a continuous glucose monitor to manage blood sugar with no multiple daily injections, zero fingersticks, and is fully controlled by a compatible personal smartphone.
Insulet has had a busy year with Omnipod 5
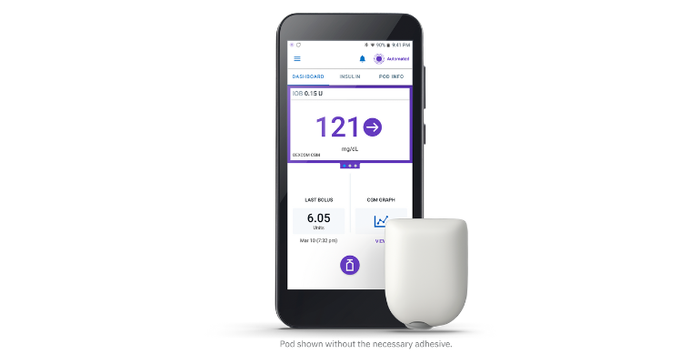
Insulet launched the Omnipod 5 system in early August, after gaining FDA clearance in January and executing a limited launch of the system. The Acton, MA-based even paid for a TV advertisement to bring more awareness to the technology.
Insulet also received CE marking to sell the device in Europe in September. The Omnipod 5 is the first CE marked tubeless hybrid closed loop system (also known as automated insulin delivery) that integrates with the Dexcom G6 continuous glucose monitoring (CGM) system to help with diabetes management.
In Europe, the Omnipod 5 can be marketed for people aged two years and older with type 1 diabetes. The system consists of the tubeless Pod enhanced with SmartAdjust technology, the Omnipod 5 controller with its integrated SmartBolus Calculator, and the Dexcom G6 CGM.
Every five minutes, SmartAdjust technology receives a CGM value and trend, and predicts where glucose will be 60 minutes into the future. The system then increases, decreases, or pauses insulin delivery based on the user’s desired and customized glucose target.
The technology had been among the most anticipated medical devices of 2021, although it didn't quite make it to market by the end of last year as previously anticipated.
Insulet also went through a major leadership change earlier this year after CEO Jim Hollingshead's predecessor, Shacey Petrovic, stepped down for personal family reasons on June 1.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
