Abbott Scores a 'Long-Awaited Win' in Diabetes
A new FDA nod marks a significant milestone for Abbott, as well as players in the automated insulin delivery space.
March 6, 2023
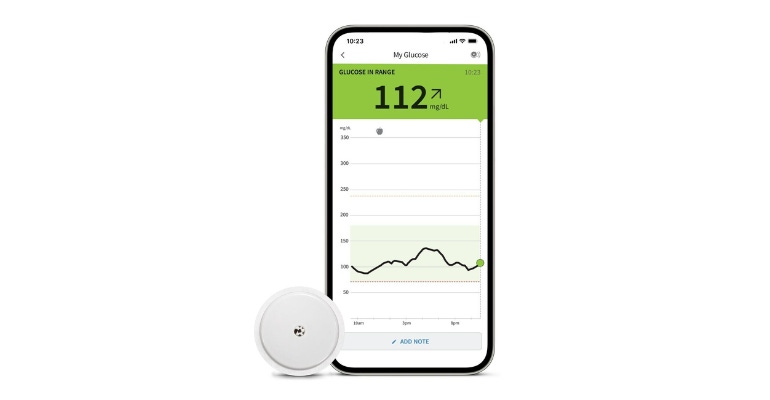
The diabetes management space just got a whole lot more interesting.
FDA has cleared Abbott's FreeStyle Libre 2 and FreeStyle Libre 3 integrated continuous monitoring sensors for integration with automated insulin delivery (AID) systems. The Abbott Park, IL-based company modified the sensors to enable integration with AID systems. The news also comes on the heels of Medicare coverage of continuous glucose monitoring (CGM) devices for all people with diabetes who use insulin.
Marie Thibault, a medtech analyst at BTIG, called the news a "long-awaited win."
"We view this as a significant milestone that unlocks a new patient population for the company," Thibault wrote in a report Monday morning, noting that about a third of the nearly 2 million U.S. patients with type 1 diabetes currently use an insulin pump for diabetes management.
AID systems are designed to help with diabetes management by automatically adjusting and administering insulin delivered by an insulin pump based on real-time glucose data from an integrated CGM device.
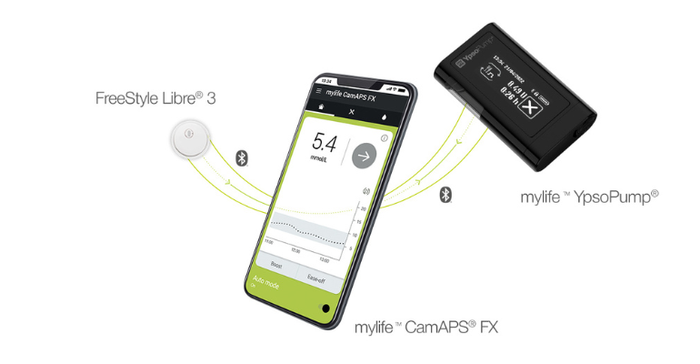
Abbott said it is working with leading insulin pump manufacturers to integrate their systems with the FreeStyle Libre 2 and FreeStyle Libre 3 sensors as soon as possible. The company is partnering with Insulet and Tandem for future integrations in multiple countries, including the United States. Outside the United States, Abbott's FreeStyle Libre 3 sensor is already authorized to work with the mylife Loop solution from Ypsomed and CamDiab (pictured below) in Germany, with additional launches in the UK, Switzerland, and the Netherlands planned for the first half of this year.
Abbott's modified sensors were also cleared for use by children as young as two years old (previously four years old) and for wear time up to 15 days (previously 14 days). The clearance also allows the Libre 2 and Libre 3 sensors (both those available today and the modified sensors) to be used by pregnant women with all types of diabetes (type 1, type 2, and gestational).
The modified FreeStyle Libre 2 and FreeStyle Libre 3 sensors are expected to be available in the United States later this year. Over time, the modified sensors will replace the current FreeStyle Libre 2 and FreeStyle Libre 3 sensors available today in the U.S. market, Abbott noted.
The milestone may be more impactful for Abbott's AID partners
While this has been a long-awaited milestone for Abbott, William Blair analyst Margaret Kaczor does not expect it to result in a material change in CGM demand or market share for either Abbott or Dexcom.
In the U.S. market, AIDs are primarily used by people with type 1 diabetes, which is already more than 50% penetrated for CGM systems, Kaczor noted in a report Monday. The analyst pointed out that most of the growth for the CGM market in the coming years is expected to come from people with type 2 diabetes (both those who are considered insulin intensive and those who are non-intensive insulin users).
The analyst also estimates that Dexcom has maintained 60%-plus market share of U.S. type 1 patients for several years despite next-generation Libre systems, citing Dexcom's "first-mover" advantage and strong clinical data. Dexcom won FDA clearance for its G7 CGM in December.
"We expect that the announcement this morning may be more impactful for AID players, such as Tandem and Insulet, as Abbott’s reach and market share — particularly internationally — can benefit these companies that have been competing with the reach and scale of Medtronic," Kaczor wrote.
Both Insulet and Tandem have made other strategic moves this year in the name of improving diabetes management.
Insulet recently acquired assets related to Bigfoot Biomedical’s pump-based automated insulin delivery and the assets of Automated Glucose Control. Insulet doled out $25 million to each company for the technologies.
Meanwhile, Tandem improved its diabetes management offerings through the recent acquisition of AMF Medical. Through this deal, Tandem inherits the Sigi Patch Pump, a rechargeable device still under development that uses pre-filled insulin cartridges.
Would a combined pump-CGM offering under one roof make sense?
Josh Jennings, a medtech analyst at Cowen and Company, raised an interesting question during Abbott's fourth-quarter earnings call. He asked Abbott CEO Robert Ford his thoughts on whether a combined pump-CGM offering under one roof would be advantageous for either Abbott or another company.
"As it relates to an all in one, I think the market has spoken in terms of the pumpers want choice," Ford said. "They want to be able to choose what is the best sensor pump combination. And so I think right now, my view on that is the consumers have spoken, the market has spoken, the regulators spoken, they want that interchangeability. And I think that our focus will be on providing the best sensor for the pump systems that are out there."
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
