MDEA 2014 Winners: Medical Product Packaging, Graphic Instructions, and Labeling Systems
MDEA 2014 Winners: Medical Product Packaging, Graphic Instructions, and Labeling Systems
June 11, 2014
Medical Product Packaging, Graphic Instructions, and Labeling Systems

|
DISK (Dispenser Integrated System Kit). Manufactured and submitted by CleanCut Technologies LLC (Anaheim, CA). DISK is thermally bonded to an HDPE backer card and is intended to provide medical device product retention and protection during packaging, handling, sterilization, shipping, and use in medical environments. |

|
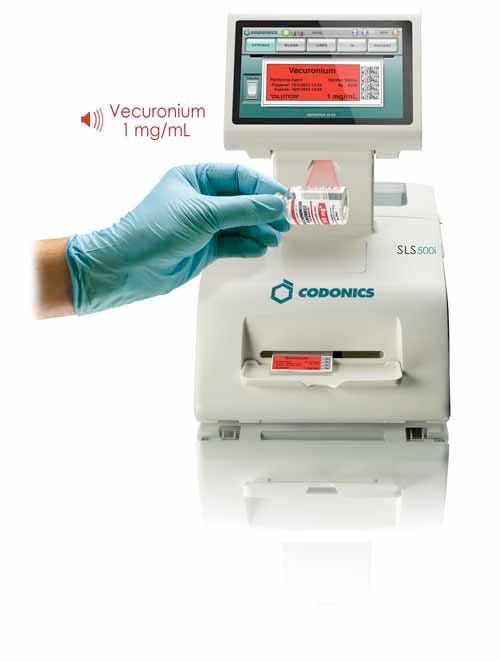
Safe Label System (SLS) enterprise-wide electronic medication preparation safety system. Manufactured and submitted by Codonics Inc. (Middleburg Heights, OH). SLS is an on-demand medication preparation-specific system that replicates pharmacy oversight at the point of care. It ensures TJC compliance with best practices and standards to improve patient safety, workflow efficiency, accuracy, and reduce drug errors. Supply and design credit to SIMS Lab and Partner’s Healthcare at Massachusetts General Hospital (Boston). |

|
SoTube/SoSafe double-sterile barrier packaging. Manufactured and submitted by Selenium Medical (La Rochelle, France). SoTube/SoSafe are two innovative solutions, nested tubes based, for implants double-sterile barrier packaging. They ensure nurses and surgeons easy handling of package and implant as well as a pioneer “No Touch” approach right up to the point of surgery. |
Other Finalists
ePAK Single-Use Delivery System featuring DVR Crosslock distal radius plating system. Manufactured and submitted by Biomet Orthopaedics Inc. (Miami).
IMS Signature Series cassettes. Manufactured and submitted by Hu-Friedy Mfg. Co. LLC (Chicago).
|
|
In Vitro Diagnostic Products and Systems | Over-the-Counter and Self-Care Products |
You May Also Like