MDEA 2014 Winners: Implant and Tissue-Replacement Products
MDEA 2014 Winners: Implant and Tissue-Replacement Products
June 11, 2014
Implant and Tissue-Replacement Products

|
Codacs direct acoustic cochlear implant system. Manufactured and submitted by Cochlear Ltd. (Sydney, Australia). The Codacs system meets an unmet patient need by mechanically stimulating the cochlea, bypassing the impaired middle and outer ear of people with severe to profound mixed hearing loss. Supply and design credit to Helbling Technik (Liebefeld, Switzerland). |

|
Eclipse soft tissue anchor. Manufactured and submitted by MedShape Inc. (Atlanta). The Eclipse offers an innovative, nonrotational approach for reattaching soft tissue to bone. It features an expandable sheath-and-bullet design that preserves tissue orientation while reducing surgical complexity and increasing conï¬dence in the repair. Supply and design credit to Enginuity Works (Atlanta) and Creature LLC (Atlanta). |

|
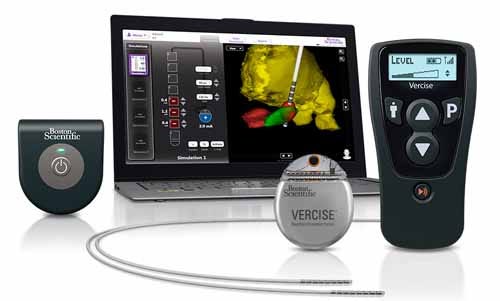
GUIDE DBS programming planning and visualization system for deep brain stimulation (DBS). Manufactured and submitted by Boston Scientific Corp. (Natick, MA). GUIDE DBS is a software program that creates a 3-D image of a patient’s deep brain anatomy and the relative location of the implanted lead. A model of the volume of activated tissue allows the clinician to intelligently optimize stimulation parameters. |
Other Finalists
Compressyn Band implant for sternal closure with Compressyn Band Tensioner. Manufactured and submitted by Dallen Medical Inc. (San Clemente, CA).
Persona, the Personalized Knee System. Manufactured and submitted by Zimmer Inc. (Warsaw, IN).
|
|
General Hospital Devices and Therapeutic Products | In Vitro Diagnostic Products and Systems |
You May Also Like





.png?width=300&auto=webp&quality=80&disable=upscale)
