Is This Device a Better Way to Lose Weight?
July 13, 2016
The new provocative device, known as the AspireAssist, is designed to provide patients who suffer from severe obesity with an alternative weight loss method--by draining ingested food from the stomach into the toilet.
Kristopher Sturgis
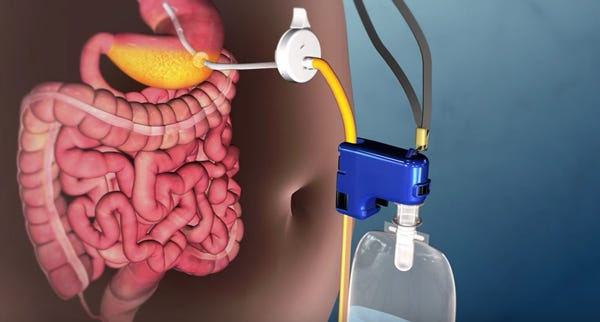
The new device approved by FDA has certainly made waves, as many have criticized the device, calling it an easier alternative to bulimia. Despite some of the criticism and its rather unconventional nature, the device has shown to be effective in the treatment of patients suffering from severe obesity.
"Any truly novel therapy is apt to be 'unconventional' at first," says Kathy Crothall, current president and CEO of Aspire Bariatrics where the device was created. "We have studies to determine if a therapy is safe, effective, and well-tolerated by patients. Like any other medical device or procedure, skeptics should look at our data. We have shown significant weight loss in all our studies, and relatively few and minor adverse events, as compared to the adverse events reported in the literature with bariatric surgery."
As for how it works, the device consists of a gastrostomy tube that is surgically implanted into the stomach. The tube is then connected to a valve in the abdomen, and the valve remains closed until it's time to drain the stomach. The device was designed to drain from the fundus, or the upper part of the stomach, where the maximum amount that can be drained is roughly 30% of the calories consumed.
Despite the potential of the device, many critics see the technology as a means to encourage eating disorders like bulimia, rather than promoting healthy eating habits.
"Throughout the studies, metabolites and electrolytes have been monitored, as have been eating disorders," Crothall says. "There has been no evidence of the development of clinically significant metabolic or electrolytic abnormality--nor has there been any evidence of patients who use this device developing adverse eating behaviors. Although the device might remind people of bulimia, bulimia is a psychological disorder associated with binge eating. Patients cannot binge with this device. If they fail to chew thoroughly and eat slowly, they cannot drain food from their stomach. "
As Crothall mentions, patients do need to chew their food at every meal if they want to "aspirate." Upon finishing the meal, patients can use the restroom within 20 to 30 minutes of eating (before the calories are absorbed into the body) to drain the food from their stomach. They then need to flush the stomach with water and drain it once more to complete the process.
To receive FDA approval for this kind of device, Aspire was required to submit safety and efficacy data from clinical studies--and much of the data from these early clinical trials has looked promising. The 2013 pilot study showed that participants who used the device for one year lost about 19% of their body weight on average--compared to just 6% for those in the control group who simply used lifestyle therapy only.
"Skeptics who worry that an unsafe device might have been unleashed to patients in the U.S. should take comfort in the review process for premarket approval of devices," Crothall says. "This review process is the most rigorous in the world, with less than 35 devices on average per year approved over the last decade."
As for the future of this device, the company is working on bringing it to market in the U.S., where it's expected to be available for around $13,000 according to Crothall. Currently the FDA has approved the device for use in obese patients ages 22 and up. These patients must have a body mass index of 35 to 55, and must show that they don't have any signs of an eating disorder. These patients are also expected to demonstrate that they have failed to maintain weight loss after trying other non-surgical methods.
Despite some of the doubts and criticism surrounding the device, Crothall remains confident that this device will have a positive impact on patients suffering from obesity, and prove to be an effective weight-loss tool in the long run.
"A number of pejorative terms have been used to describe this therapy," she says. "If this device were to treat some other condition other than obesity, say for example cancer, would one be so quick to use these pejorative terms? I doubt it. Obesity is a stigmatizing disease, associated with reduced quality of life, significant morbidities, and reduction in lifespan. This therapy is minimally invasive, safe and effective, and reversible."
Kristopher Sturgis is a contributor to Qmed.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
