How Medical Device Engineers Support Innovation in Unexpected Markets
Engineers and designers are trained as problem solvers, but disease management presents unusual challenges. In an upcoming Center Stage session at MD&M West 2021, Product Creation Studio CTO Scott Thielman will explore recent, real-life examples of medical device developers applying their skills to unique and meaningful opportunities. In this article he previews one of the stories he’ll share on the delivery of next generation nucleic acid vaccines—technology that may help to squelch the next pandemic. The talk will also include takeaways on innovation management and the application of a development process to support translational research.
August 5, 2021

Vaccine delivery is suddenly a hot topic in bioscience and healthcare. At Product Creation Studio, we’ve had a front-row seat to influential research in this field. While we are not experts in vaccines or microbiology, we do know a thing or two about user-centered design applied to drug delivery and other healthcare workflows. We often support innovative researchers as they translate their discoveries into products with a commercial vision. I realized that stories from this type of work might be interesting to other medical device experts. While these projects are not without challenges, who wouldn’t want to support visionary efforts with a huge potential impact on healthcare? I decided to share some of our stories with the hope that they will be instructive for others involved with scientific translational work.
Delivering Genes
Deborah Fuller is a microbiologist at the University of Washington focused on next-generation vaccines and vaccine delivery. Pre-pandemic, she had been telling us about the potential of nucleic acid vaccines (including mRNA), but it honestly seemed like a long way from having real impact. As COVID raged, I had the chance to discuss with her the differences between self-amplifying RNA, DNA, and mRNA vaccine technologies and realized how much of an authority Deb is in the field of vaccine delivery.
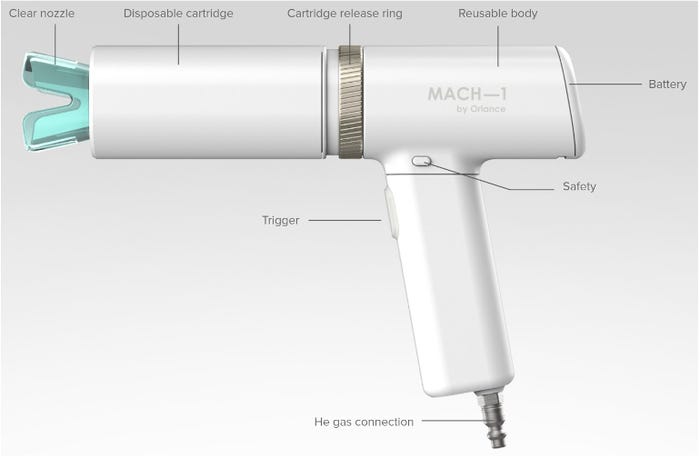
As a mechanical engineer, I found it very cool that at the center of much of her research was an unusual electro-mechanical device called a gene gun. This novel delivery technology, under development at her spin-out company Orlance, uses a controlled blast of pressurized helium to carry tiny, specially treated gold particles toward the target tissues. Basically, they coat the gold particles with a dry form of the nucleic acid and use the inertia of the dense gold to deliver the particles directly into the cells. This offers a very flexible delivery platform with low dose requirements, room-temperature doses, and needle-free painless delivery.
But this is not the ubiquitous and familiar syringe format; there are new systems and workflows to develop along with the vaccines to leverage the technology. Our team has supported their efforts to achieve the desired pneumatic pulses but also to help them think about the workflows and what a clinical gene gun might look like. It will take the Deb Fullers of the world to create the next generation vaccines, but it will be device designers and engineers who translate them into commercial solutions.
Design and engineering firms like Product Creation Studio generally work with a wide range of clients. Support for innovation in translational science is not without challenges; projects often pause for extended periods between rounds of funding from government grants or contracts. It is not unusual for the innovation to precede an understanding by the commercial market it is intended for. But theses translational projects are rich in opportunities for medical device developers to have significant impact. Team motivation is rarely a problem when the goal is blocking an outbreak to save lives.
To hear more stories, join us August 12 at 11:00 am for the Center Stage session at MD&M West 2021, “From Gene Guns to Freeze Spray: How Device Engineers Support Innovation in Unexpected Markets.”
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
