How to Make a Disruptive Device That Wins FDA Approval
February 4, 2015
Agamatrix and Misfit cofounder Sridhar Iyengar has some advice for medtech professionals hoping to win FDA's support for innovative new medical devices.
Brian Buntz
|
Sridhar Iyengar is the cofounder of Misfit and Agamatrix. |
How can a medical device company create an unprecedented product that can win regulatory approval? Qmed recently had the chance to interview Sridhar Iyengar, the cofounder of Agamatrix (maker of the iBGStar, an iPhone-compatible blood glucose meter) and Misfit (a wearables and smart home company).
The notion of disruptive innovation, in which a seemingly simple product ultimately becomes more powerful and disrupts an established market, has become one of the most popular business theories in the past couple of decades. The idea, popularized by Clayton Christensen, gives a theory to help explain the phenomena we see repeatedly: Netflix helped spell the end for video rental stores and Amazon has helped disrupt the businesses of booksellers and, most recently, RadioShack. The traditional music industry, still reeling from the popularity of digital music, is again being disrupted by Spotify--a subscription music streaming service that gives users access to more than 15 million songs.
So what are some examples of disruptive innovation that are applicable in the healthcare technology realm? And what is the best way to go about creating them? Iyengar tackles these questions and more in the following Q&A.
Qmed: What are some good examples of disruptive healthcare products and how do you see such products from a regulatory perspective?
Iyengar: Although it's not a medical device, 23andMe is a great example of a disruptive healthcare product. They approached a market where the regulatory guidance was very unclear. With a genetic test like that, you are not telling the patient that they have a disease or not, but that they have potential risk factors based on their genetic profile. And it is a laboratory test; it is not a consumer diagnostic. They are actually not doing the test; they are just marketing it. They are using the Illumina service on the backend.
23andMe crosses several different areas so it is very unclear. My personal feeling was that the only way they were going to get clarity is to go and make their best possible product and put it into the market and engage the FDA that way. I think they have done the right thing. And ultimately, FDA said: 'sorry, stop marketing this as a diagnostic.' But to actually try to figure out what the FDA wants ahead of time is tricky when you are making a new product.
The regulations are pretty clear about a small number of things. But they are highly unclear about a large number of things. If something has already been in the market and there is a history of its use, the FDA tends to have very clear guidelines.
But once you break out of that mold, the FDA doesn't have guidelines. So basically you have to go do it. You have to use your best judgment, and make a product, go to FDA, talk to them, and get them on board. But when you talk to the FDA, and you say: 'hey, I am going to make this. Do I need your clearance?' It is highly unlikely that they will say: 'Go ahead and do whatever you want.'
A lot of the Silicon Valley companies will approach them in that way or will just not approach them at all.
Qmed: How about a good example of a recent innovative medical technology that has won FDA approval?
Iyengar: Dexcom recently won FDA clearance for their SHARE mobile control app for their G4 continuous glucose meter. That is a huge win for them. That was a journey that took them half a decade to complete.
Qmed: Many medtech innovators end up giving up on unique medical device projects because they fear they will never get FDA approval. What message would you have for people like that?
|
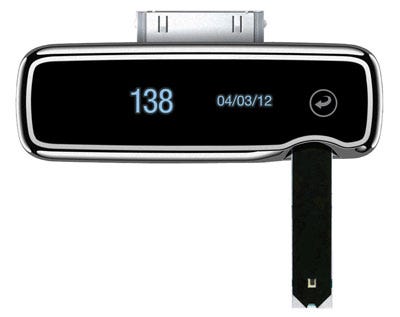
The iBGStar blood glucose meter from Agamatrix was the first glucose meter that was compatible with the iPhone. |
Iyengar: Contrary to stereotypes, the FDA is not unreasonable. They are actually extraordinarily reasonable. I advise startups to not be afraid of the FDA. First of all, having regulatory approval is a great barrier to entry to have. And all the FDA is saying is: 'If you say your product does x, y, and z, just substantiate the data in a way that gives the general public comfort and a reasonable assurance that what you are doing is safe and effective.'
I'll give you a funny example: With the IBGStar, when we first did our submission, the FDA came back and said: 'Your product is all well and good but you are relying on people's ability to download the app and install it on their iPhone before they can use your product. How do you know people can do that?'
They said: 'We are not doubting that people can download apps. All we are saying is that you don't have the data to demonstrate it.' That is when the lightbulb went off: we just needed the data to show our product does x, y, and z. They were just telling us that our submission was incomplete. So we did a study so we could have a dataset that people could download the app.
If you look at it from FDA's standpoint, if there is ever an adverse event because somebody did something incorrectly, for whatever reason, they don't want the manufacturer to be liable for that. They are basically protecting us.
In terms of disruptive technologies, by their very nature, they will be things that haven't existed before. So the best you can do in terms of working in the regulatory environment is to definitely have the conversation with FDA. The FDA wants new technology on the market. But it is up to the manufacturer or the inventor to come up with the risk profile to show what the risks are and how to mitigate them. Don't rely on the FDA to tell you that. They are not consultants. Don't ask them to tell you what is wrong with your product. They are here to basically establish whether you have done a good job with your risk assessment and risk mitigation. They are not the experts when it comes to the risks of your device--you are as the manufacturer.
I think that is where a lot of disconnect happens with new startups who are working in the regulatory space is that they often think the FDA should tell them what to do and they are not going to tell you that. The burden really falls on the developers to dot your I's and cross your T's. If you take that attitude, working with the FDA is actually pretty easy.
Qmed: Another complicating factor is the traditional boundaries between consumer and medical devices are blurring in a lot of cases.
Iyengar: It is blurring. And I don't think it is going to ever get clearer. If it is not the latest FitBit, which has heart rate monitoring capabilities, it will be something else.
For now, FDA is largely saying if you monitor heart rate, it is no big deal. But let's say somebody says: 'wait a minute. With this kind of a heart rate, I may have some risk of fibrillation or arrhythmia.' If there are a bunch of disclaimers, then the new FitBit's heart rate feature wouldn't fall under FDA's purview because it is likely not going to be used in a medical context. But if enough people start making medical decisions based on that data, that is when it is going to fall under FDA's jurisdiction.
Even if a patient thinks they have arrhythmia from using a heart rate monitor, if the action they take is to see a doctor, it is no big deal. If the action they take is to get a medical procedure done based on that, then it is a big deal. It comes down to how are people going to use your product. And to some extent, manufacturers don't have control over that but they have to plan for it in their risk assessment.
This is kind of what happened with 23andme. It was high profile folks like Angelina Jolie making headlines about breast cancer and genetics. If something like that happened with consumer fitness devices, it could move them to the medical realm. It can be a completely unintended off label use, but that still is a risk, and you need to address that as a manufacturer.
23andme's original argument was: 'we are not going to say you are going to get this disease or get cancer. We are just saying you have this gene.' The FDA shot back, saying: 'that might be technically correct but if you give this information to a person, they are likely going to make medical decisions based on your information. Disclaimer or not, they are going to use the information in a way that is medically relevant. So the burden falls on you to make sure that if you provide this information, it is correctly actionable.
Qmed: Simplicity has long been a popular design principle with saying like "less is more." How does that apply to medical device design?
Iyengar: If it is a true to form medical device, you don't have to make it so simple. Depending on the complexity of it, you will either get trained how to use it in an outpatient setting or it will be a professional product.
But if I go back to my experience at Agamatrix, I realized you can't design one product for everyone. So what we ended up doing is, even with our same core technology, we designed three or four different products--each with different levels of complexity. We had the simple version and the pro version. You could enable the other functions if you want, but all of that was hidden so the simplest version was on top. If the power users wanted additional complexity, they could do that behind the scenes. That is one way to do it...
The other way is to just have different products. We had a simpler low-cost product that didn't have a lot of the higher features and then we had more complex products with more of the features and we differentiated that with the packaging.
But when it comes to consumer products, complexity will kill. Even if you have great technology and features, unless it is hidden or unless it is accessible through a pro version, it is not going to succeed.
People want a simple experience. That was the driving force behind the Misfit Shine. At the end of the day, people didn't actually want a number on their wrist. That is what we found. People just wanted to know: have I done enough or not? If you needed more stats, you can go to your app, you can look at all of that. But we offloaded complexity onto the app and kept the wearable extraordinarily simple.
Again, that is something we learned from the IBGStar: make something that looks beautiful that gives people an emotional tie-in and make it as simple as possible to use. The IBGStar was a device that could be used by itself. Even though it could plug into an iPhone, you didn't need the app to make it work. We wanted people to use the app but it turned out that most of the people never left it plugged in. It was such a convenient device, they kept it in their pocket and used. Maybe once a week, they would plug it in and view their results for those seven days.
One of the things that we learned at Agamatrix and Misfit is that people don't like choices. This is something that Steve Jobs embraced. You don't give them people choices: you give them just those few selections that are good for them. Yes, you can be presumptuous and say: 'we know what is better for the user.' But at the end of the day, you overwhelm anyone with lots of lots of choices. They will be like a deer stuck in the headlights--afraid of making a mistake.
Qmed: When it comes to medical devices, how does simplicity of design relate to usability?
Iyengar: Risk mitigation remains a challenge for the medical designer. You have a minimum amount of information to convey yet you need to convey it in a way that doesn't cause the user to fail the usability aspect of using the product.
That is a big problem. A lot of times you can get the underlying technology to work really well, but you fail on the usability side of things.
In the consumer world, beta testing can help with this. But unfortunately you can't beta test medical devices in the same way because it is tricky.
In the end, you just need really good people who can meet the health and safety risks versus the financial risks because you can always test and design forever and never get a product on the market. But this is where good judgment comes into play. You need somebody who has lived this to say: these are risks that are acceptable to take and these are risks are unacceptable to take.
A number of years ago, someone from the Department of Homeland Security said something to the effect that "For us to be successful, we have to win every single battle. But for terrorists to be successful, they just have to win one battle."
That is the same way I look at the medical device world and regulations. For a product to be safe, it has to win every single time it is used. For a product to be unsafe, it only has to fail once.
Hear Agamatrix and Misfit cofounder Sridhar Iyengar speak on disruptive medical device design at on February 10 at MD&M West in Anaheim, CA. |
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like