How 3D Printing Enabled a Giant Leap Forward for Ostomy Patients
In this Behind the Design story, learn how 3D printing made manufacturing the OstomyCure Ties implant much more feasible.
January 26, 2023

Materials, 3D printing technologies, and applications are all poised to make meaningful advances in 2023 and beyond. Already there are medical devices in development and on the market that simply wouldn't be viable products without advancements in 3D printing. The Ties implant is a perfect example of a device that would not be where it is today without 3D printing.
The Ties implant, being developed by Oslo, Norway-based OstomyCure, is a titanium port designed to be implanted within a stoma. It is intended to be used with an attachable lid to effectively open and close the stoma. The idea is that patients can empty their stoma at their own convenience as opposed to it continuously emptying into a bag, causing discomfort, noise, smell, and inconvenience.
The company has been developing the technology for well over a decade and while it isn't perfect (some patients still experience leakage around the stoma) the technology represents a giant leap forward for patients living with a stoma and relying on an ostomy bag.
OstomyCure recently reached a crucial milestone in its pivotal clinical trial: 20 patients have been implanted with the device in the UK, Sweden, Poland, Austria, and India. The company said the patients will be monitored for six months to fully assess safety and performance of the device. The data from these patients will then be included in the company's submission for a CE mark under the new Medical Device Regulation (MDR) in the European Union.
"Completing this milestone for our pivotal clinical trial is a crucial step in bringing this novel device closer to regulatory clearance and introducing it to a large patient population urgently in need for solutions to improve their quality of life," said OstomyCure CEO Johan Jarte. "We will quickly compile the data ready for our EU regulatory submissions. We are incredibly grateful for the commitment and enthusiasm of patients and clinicians who have taken part in the trial."
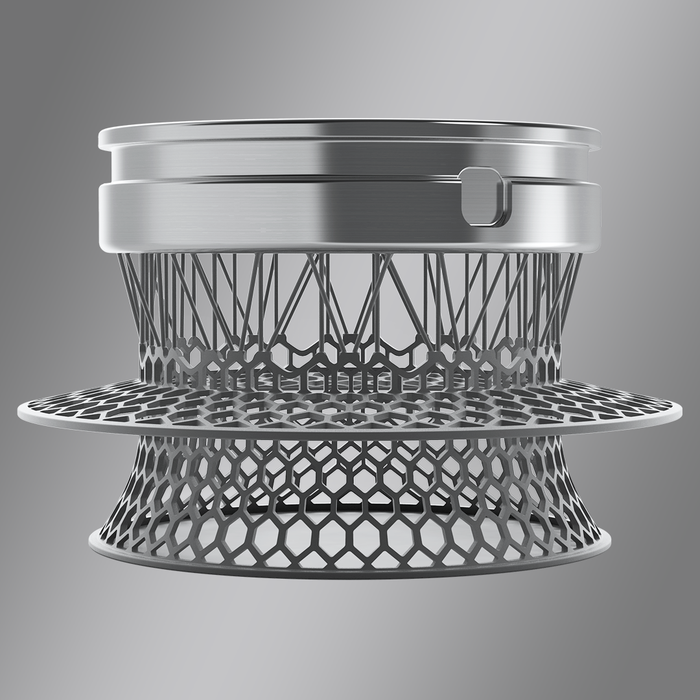
'Without 3D printing we would not have been able to come this far'
Early iterations of the Ties implant were made using conventional manufacturing equipment, Mats Cardell, chief technology officer at OstomyCure, told MD+DI.
"It was very complicated, very expensive technology, and still not a perfect design," Cardell said.
So, the company began 3D printing the implant in 2012, which was still the early days of metal 3D printing, he said.
"Then we could optimize the design," Cardell said. "There is much more freedom in designing things in 3D printing."
With 3D printing, the surface of the titanium implant is also better for tissue in-growth because it is a rougher surface, Cardell said.
"I'd say without 3D printing we would not have been able to come this far," he said. "It has been a cornerstone for us."
Behind the design of the lid
The company's design and engineering focus in the early years was on the implant itself, making sure it was safe and effective. At that time, the thinking behind the lid portion of the device was simply that it needed to be a cap. So, the first-generation lid design was rather basic.
A second-generation lid was designed which was better than the first, Cardell said, but the patient had to remove the lid to empty their stoma. The problem with that is there tends to be pressure inside the stoma and as soon as the lid was removed stool would spurt out all over the person's hands.
"So, it wasn't very user friendly even if it was safe and working really good as a lid," Cardell said. "Then we decided to go for a third version of the lid which would have a little hatch in it so you can actually open the hatch while the lid was still on, and this was possible to do from the outside. First you put a bag on top of the lid and then from outside the bag you can open this hatch and you would empty the stoma into the bag."
That was "nice thinking," he said, but it still didn't work as well as the team had hoped.
"So now we're into the fourth generation of this where we have combined the safe and nice design from the [second generation] lid but it has an opening so you can open it into a pouch without soiling your hands," Cardell said. "... We're quite happy with this design."
As the company recruits more patients into the clinical trial and gathers additional user feedback, Cardell said the lid design will likely evolve further. He also acknowledged that every patient is different and patient preference is likely to differ from one user to the next, so the company is thinking about additional accessories to accommodate those various user preferences.
The lids can be washed and reused but are intended to be replaced weekly.
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
