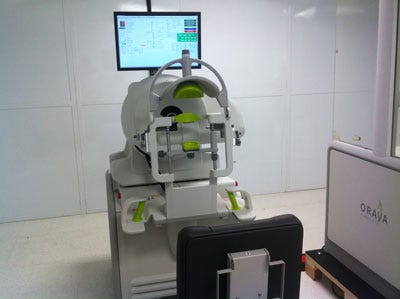
A Disruptive Device That Treats Vision Loss
August 18, 2015
The Silicon Valley startup Oraya Therapeutics offers a case study in innovation in the medical device sector.
Brian Buntz

Oraya Therapeutics (Newark, CA) is unique from a technological perspective. The private company, which won silver at the Medical Design Excellence Awards this year, has developed a noninvasive therapy for wet age-related macular degeneration--one of the most common causes of blindness. The company's IRay radiotherapy system delivers a precise dose of radiation noninvasively to the eye in an outpatient setting. The radiation is thought to have an anti-inflammatory and anti-fibrotic effect on the macular, the pigmented region located near the center of the retina. While the Oraya device doesn't do away with the need for the traditional treatment--anti-VEGF injections, it does diminish the need for them significantly.
"The IRay radiotherapy system replaces the need for regular doses of an expensive and invasive drug with a radiation therapy that is less costly and has better outcomes," says Bill Evans, principal and founder of Bridge Design (San Francisco), which assisted in the design of the device.
Evans says the technology is a good example of a disruptive medical device--one that dovetails with the current cost-conscious demands from hospitals and clinics in the United States.
Jim Taylor, CEO of Oraya Therapeutics, says the company, has sought to address these demands. "Nobody right now anywhere in the world (and certainly outside of the United States) is looking for technology that cost more and provides modest or no significant patient benefits," Taylor says. "In that regard, we provide something that is better for the patient, easier for patient, and better for the insurer. It also unloads a lot of capacity constraints that treatment facilities are going to need to address as the Baby Boom generation moves into diseases of chronic aging."
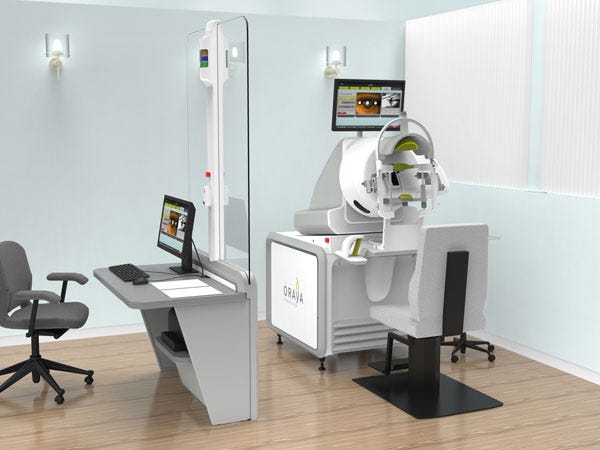
Founded in 2007, Oraya had the strategy of defining a patient population that has the best possible chance of benefiting from a novel integrated treatment. "I think the promotion of individualized medicine has been overdone; it is a bit of an overpromise. But when you look at patient segments and figure out how to best put them into categories of integrated therapies, and the best potential for a given outcome, that is where I think the future of medicine is going."
Taylor also observes that, as the population of developed nations faces an uptick in chronic disease, they are also wrestling with low compliance of treatments requiring chronic interventions. "We have a one-time noninvasive approach," he says. "If you look at diabetes or blood pressure, glaucoma over the past 20 years, one of the biggest problems in treating them has been patient compliance, which sometimes is in the order of 50%."
When asked to speculate why MDEA jurors selected the company's technology for a silver award, Taylor imagines that jurors were impressed that "the concept itself was a solution to a problem that people have been trying to figure out for about 20 years."
"At the core of that design is a very innovative approach in how to solve a problem that people hadn't been able to figure out for two or three decades: how to deliver radiation to the back of the eye in a very precise and well targeted manner," Taylor says.
|
The IRay device has a contemporary design. |
It also helps that the device has a sleek appearance, which was a challenge to create given the regulatory constraints the company faced during the development of the device. "Once you have established a core platform methodology, and have done some early clinical work to demonstrate the safety and efficacy of that implementation, it is very difficult to go back and change its core principles without going back to the beginning of the regulatory process," Taylor says.
For that reason, refractive laser systems used therapeutically use a platform that has been around for years. "Any time you go back and say: 'Hey, we have a great idea on how to totally change the energy delivery,' it not only changes the design of the whole product but you have to go all the way back to the clinical trial," Taylor says. "One of the constraints on our design with Bridge Design and ourselves is that we could not go into the core energy delivery, the targeting precision aspects of the robotic system for the energy delivery system, or the source system. Those things became fixed elements within the design, which made our design job harder."
The two companies each came up with ideas on how to optimize the look and feel of the device. "One of the key elements of Bridge's design was how we can give this a modern look and feel, a good user interface, a good patient experience, but not go all the way back and start all over again," Taylor says. "I think the challenges of the design were how to take a, for all intents and purposes, an advanced prototype--at least at the core therapeutic delivery level--and update the design into a sophisticated, easy-to-use piece of electronic medical equipment," says Taylor. That was no easy feat. "It is pretty complex to determine what we can touch, what we can't touch, and how can we make sure it is designed in an integrated fashion as opposed to stitched together haphazardly."
Learn more about cutting-edge medical devices at MD&M Philadelphia, October 7-8, 2015. |
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like