Designing Devices That Patients Want to Use
February 10, 2015
Guess how much the problem of noncompliance costs the United States each year? $100 billion.
|
Sridhar Iyengar |
Brian Buntz
OK, everyone knows that fostering patient compliance is a huge problem. Less certain is how to address it.
Solving that problem is a matter of understanding users' psychology and emotions, says Sridhar Iyengar, CTO of Misfit (Burlingame, CA), who delivered a talk Tuesday at MD&M West in Anaheim, CA. The best way to do that is through an anthropological approach rather than focus groups or surveys. It is better to be embedded in a user's environment to get a sense of their daily routines.
Iyengar recalled that while working at Agamatrix, the company sent patients home with disposable cameras and had them monitor how they used glucose meters and then used that information to design products that were successful in getting patients to continue using them over the long haul.
To accomplish that objective, a product ideally needs to be easy to use, relevant to life, generate an emotional bond for the user, be enjoyable to use, and truly be meaningful. The ease of use aspect relates to human factors, while the "relevant to life" attribute ties in to safety and efficacy--FDA's criteria for market approval of medical devices. The final three attributes--emotional bond, enjoyable, and meaningful--all relate to patient compliance.
|
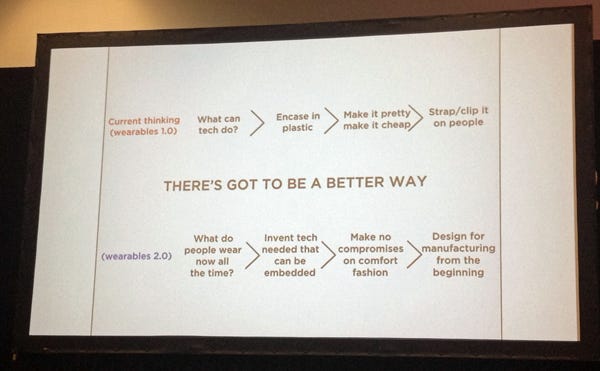
A chart from Iyengar's talk at MD&M West. (Photo by Shana Leonard/UBM Canon) |
Iyengar learned the importance of good design, which considers all of these criteria, while working at Agamatrix, which is best known as the maker of the IBGStar, an iPhone compatible glucose meter. The company received letters from pleased parents who reported that their teen and preteen children loved the device. Some of them payed for the device with their own allowance while others reported that they were no longer embarrassed to have diabetes. Some showed of their iPhone-compatible device at school.
Agamatrix had succeeded in making a medical device that stood out in terms of fostering user compliance.
The founders of the company (Iyengar and Sonny Vu) took the lessons learned from Agamatrix into the consumer wearables market several years ago by founding Misfit Wearables (now rebranded as Misfit). Although there were already a number of fitness trackers on the market, they had some key flaws. One of them was the hassle of charging them periodically. The other was related to aesthetics: they were clunky pieces of plastic that either strapped to clothing or were worn around the wrist.
Still, those early products had an enthusiastic, if limited user, base. And the athletes who were spotted wearing one or more wearable devices had no qualms in looking like the Iron Man (or Woman).
 To differentiate their products, the company developed a product that did not need to be frequently recharged, but could last for several months with a single battery.
To differentiate their products, the company developed a product that did not need to be frequently recharged, but could last for several months with a single battery.
Just as important, however, was the topic of aesthetics. Rather than having a user look like a Quantified Self-inspired triathlete, Misfit wanted to make their product invisible. "Lots of people don't want to advertise that they are trying to lose weight," Iyengar said.
One example of a super-user friendly device is profoundly low-tech: the Band-Aid. It has become a staple product, known by a brand name in the United States, because of its ease of use and relevance to life, Iyengar noted.
Another product in the fitness realm that succeeds is Zombies, Run!, "It is very simple," Iyengar says. "You start running with an app and earbuds, and when you slow down, you hear zombies chasing you."
You don't have to be a maker of a low-tech medical device or a fitness app to design a rewarding product, however. Iyengar pointed to the success of Insulet, which makes a fully automated insulin pump that is easy to use. "It takes away complexity," he explained, and helps engage the user.
Hear from other medical device experts in the MD&M West conference lineup in Anaheim, CA, held February 10-12. |
Brian Buntz is the editor-in-chief of MPMN and Qmed. Follow him on Twitter at @brian_buntz.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like