Design Excellence Under the Hood
For some award-winning devices, their software, wireless connectivity, or other unseen features helped pushed them to the top.
April 1, 2007
MDEA 2007
More and more, devices are driven by software that works quietly in the background, increasing safety and ease of use. As such, software is often a fundamental part of a device's functionality. And so, excellence in design requires more than just a pretty package. The Medical Design Excellence Award (MDEA) winners described here were among those that represent not only excellence in outward design, but also software or system integration—ensuring safety or reducing errors.
“To some extent, the safety element integrates the…concepts of aesthetic design, simple execution, and intelligent process,” notes Ogun Gurel. Gurel, who is chairman of Aesis Research Group, served as a juror of the awards. “For example, devices in which the software element helped to minimize or even eliminate errors scored very high among the judges,” he says. “It is clear that medical devices are becoming increasingly based on informatics, and this trend will certainly continue.”
The Pinnacle of Success
|
Custom-designed software is crucial to the Pinnacle total parenteral nutrition management system, manufactured by B. Braun Medical Inc. |
Among the software-driven winners was the Pinnacle total parenteral nutrition (TPN) management system, which was manufactured by B. Braun Medical Inc. (Bethlehem, PA). The system includes a custom-designed software package that assists the user with the entry of TPN orders. The software performs the conversion calculations and checks limits.
“Our TPN manager differs from previous generations in many ways; however, the greatest differentiation is through the management and processing of TPNs,” says Mike Golebiowski, product director at B. Braun.
TPN formulations, he says, can be very complex, requiring numerous time-consuming calculations for a single order. “Every facility has a different approach to managing TPNs. Hospital pharmacists are receiving more work orders than in previous years, and compounding demands have far outpaced available technology, he explains.
“Our browser-based platform automates calculations. It streamlines the process from order entry to infusion, from physician to pharmacist to patient—standardizing the entire process. Existing technology focuses on the pharmacy, mainly providing software for the pharmacist and connecting it to the compounding device,” explains Golebiowski.
The jurors note that the integration of the software was crucial to its utility and value. “What distinguishes this product is that, from a collection of stock solutions, the user can simply and conveniently prepare the custom nutrient formulations for a particular patient,” says juror Craig Jackson, PhD. Jackson is president of Hemosaga Diagnostics Corp. “The software to examine the selected composition for incompatibility is a very good component of human factors engineering.”
According to Golebiowski, the system encompasses and provides the entire TPN management process. It creates seamless connectivity and management of the full TPN order, thereby improving efficiency and safety.
“Typically, a TPN order can include from eight to 18 individually dosed medications,” says Golebiowski. “Each of these medications may be entered in a number of different formats, sometimes within the same medical facility.”
Golebiowski says a primary objective of the software was to simplify this ordering process while reducing the potential for user-entry error. What this means, he explains, is that the complexity of TPN ordering is handled by the internal software while making the user-entry process as simple and as straightforward as possible.
“The product's compound compatibility checking system should take a lot of the worry out of preparing TPN fluids,” says juror Michael Wiklund. “Knowing that the system checks for mismatches as well as other preparation errors, users should be able to perform arguably tedious tasks effectively at an increased pace.” Wiklund is president of Wiklund Research & Design.
Calcium and phosphate (Ca/P) incompatibility is a serious concern in TPN formulations given its potential to cause serious harm or even fatality in patients. According to Golebiowski, current software platforms have hard limits and warnings for recognizing that the calcium and phosphate are incompatible at a certain dose, but fail to identify and recommend clinical adjustments or to do so in a graphical format. B. Braun worked with drug compatibility expert Lawrence Trissel, RPh, to incorporate his Ca/P studies into the Pinnacle.
“Ca/P compatibilities to reduce potential errors is a large step forward and will speed up the preparation time of TPN solutions,” notes juror William Schneeberger, MD. Schneeberger is a cardiothoracic surgeon at the University of Cincinnati Medical Center. “This eliminates the need for multiple entries of the order, because it stays in the same system until it is given to the patient,” he says.
From a software development perspective, the company had to take a number of factors into consideration. For example, the developers had to consider the requirements of a complex order that could be entered at several different types of facilities (e.g., hospital pharmacy, home-care pharmacy, etc.), within one facility (e.g., a single hospital), or across a number of different facilities (e.g., satellite pharmacies, physician offices, etc.).
According to Golebiowski, TPN ordering methods have remained relatively constant over the years. The Pinnacle embodies many of the basic concepts of previous and existing TPN systems. However, it uses all-new technology. “Every software component of Pinnacle has been built from the ground up—from the embedded firmware to the browser-based TPN order entry interface,” he says. “At the beginning of the project, it was unclear where technology would be in five years, so Pinnacle was built to be able to run on Windows, Linux, or other server platforms. The Pinnacle system is designed to endure, and take advantage of, the inevitable evolution of the hardware and software that Pinnacle uses.”
|
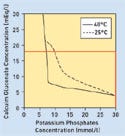
Figure 1. (click to enlarge) B. Braun's software indicates the level of compatibility of calcium and potassium phosphate. |
“When a physician or pharmacist creates an order,” Golebiowski says, “the software automatically looks for a matching clinical study.” This replaces the manual search for clinical studies after a hard limit is presented. The software identifies a study and plots the Ca/P information (see Figure 1).
From there, he says, the physician or pharmacist could comfortably make necessary adjustments to maximize calcium levels while keeping the Ca/P compatible in the formulation. Maximizing calcium is critical in certain patients, such as neonates, he explains, so many physicians or pharmacists push Ca/P to the limit.
In addition, users would receive more than a standardized graphic presentation of the research results. They would also have access to examples of the principal factors that influence the precipitation potential of parenteral nutrition solutions. As the physician or pharmacist adjusts the Ca/P, new plots would appear along the graph.
One of the first issues the company had to address, says Golebiowski, was the software language that the product should be written in. Moreover, he says that the company had to address this challenge several years ago. B. Braun decided to develop the product to be used with a browser-based interface that could be accessed via a network.
“Like with infusion pumps, TPN ordering is a site of potential medical errors,” says juror Gail Baura. Baura is a professor at Keck Graduate Institute. “All of the software in this device, not just the Ca/P Check software, assists in making TPN management efficient, from bar code verification to quality assurance checks to solution compatibility.”
Infusing Software into the System
|
The Symbiq infusion system by Hospira is designed to make patient care easier and safer. |
Infusion pumps have had their share of bad publicity recently. But one of this year's award winners is an infusion pump that is designed to make patient care easier—and safer. And software was a critical component. The Symbiq infusion system by Hospira (Lake Forest, IL) uses the company's MedNet safety software to apply institution-defined drug dosing limits to improve patient safety. The device is a next-generation general-purpose infusion pump that delivers fluids, medications, blood, and blood products parenterally and intravenously.
“Historically, software was a relatively minor piece of putting together an infusion pump,” says Steve Pregulman, global medical director for device development at Hospira. “It was really with Symbiq that Hospira embarked on a big [software development] effort.” Pregulman notes that he was brought in as global medical director, in part, because of his background in healthcare software.
“Without the software portion, you really couldn't do the drug libraries—at least you couldn't tailor them to institutions. You'd have to just pick one generic library for everybody, which is what infusion pumps have done traditionally.” And then, he says, the drug lists were provided without any kind of drug limits. The Symbiq software not only allows the creation of these libraries, but also the update of libraries as new drugs come on the market, old ones are taken off, or the institution decides that it wants to change the safety limits.
The safety software was certainly critical to its selection as a winner. Human factors also played a part. “I would link safety software to human factors engineering in devices such as infusion systems,” says juror Jackson. “Dosages that may involve somewhat complicated calculations need backup,” he says. Given the adverse events associated with infusion pumps, he thinks the software that provides the protection for this pump is important. “In my view, it is a necessary component in such devices.”
According to Pregulman, the Symbiq is dramatically different from traditional infusion pumps. “What is unique about ours is the large touch screen, which to my knowledge no other such device has.”
“The attention to detail like large lettering, touch screen input, and easy removal and placement on poles will set a new standard in the world of infusion devices,” notes Schneeberger.
“The product's safety software was certainly important to its selection as an award winner,” says juror Baura. “As we all know, drug infusion issues seem to be one of CDRH's pet peeves these days. And with the recent publication of the Institute of Medicine's Preventing Medical Errors reports, there is definitely an increased focus on safety.”
Another unseen feature—but one that is getting more attention all the time—is wireless capability in devices. “The greatest technological challenges we had were actually around the implementation of the wireless connectivity in the hospitals,” says Pregulman. “Wireless networks are relatively new—certainly within the last 10 years and, for many hospitals, within the last five.”
He says the challenges came in different forms. “There isn't any standard recipe or road map for hospitals to follow. They've all evolved differently in terms of the layout of their wireless network access points and in terms of the nature of the wireless technology—802.11a, b, or g,” he says. “We also discovered they all have their favorite encryption specifications, so we can't go out there and have [the infusion pump] tested on one type of wireless network. In essence, we have to test it in every single hospital.”
Pregulman says it's the customers who are demanding smart pumps. “We want to develop some good return-on-investment stories when customers look at the cost of devices versus the potential cost of errors that are prevented,” he says. “But,” he adds, “those data are rudimentary, at best, today. So most institutions are making their decisions simply based on the reduction-in-errors side of the equation, which is quite demonstrable.”
“We did a lot of user-needs assessment early in the project, and indeed we did this in response to the aging nurse workforce. They need a big screen so the font can be bigger. And they need the screen brighter so they can see it from a distance,” explains Pregulman. “A lot of the design was built around the fact that nurses are busier, older, and have more patients to care for than ever.”
Wiklund says the judges were also encouraged to see that Hospira invested considerable effort into ensuring the pump's usability along with the rest of the software user interface.
“It's one thing to make a feature-rich infusion pump, but another to ensure the device's overall usability in the context of real-world use. Hospira's documentation suggests they have achieved both goals through the application of good human factors practices, as described in AAMI's standard on the topic,” says Wiklund. “I was particularly impressed that the system was cited by the Human Factors and Ergonomics Society for excellence in user-centered design.”
Wiklund notes that the considerable real estate dedicated to the display facilitates more-complete communication between the user and the device compared with devices with smaller screens. “I view the Symbiq infusion system as a step forward in terms of giving users a large, color screen through which they can see clinical information and procedural guidance,” he says.
Pregulman believes the future of infusion pumps is in their integration with other devices—not just for the sake of integration, he says, but for better patient care. For example, he points to devices that monitor respiration.
“There are efforts under way to integrate certain kinds of monitors with infusion pumps. By integrating a device that says a patient isn't breathing effectively and then stopping infusion of a medication that could suppress respiratory effort makes complete sense,” he says. There are a lot of other drugs delivered by infusion that, based on the output of some monitoring device, it would be prudent to make a change to the delivery of that medication, says Pregulman.
A Clearer View
|
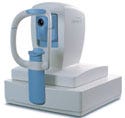
Jurors called Optovue's RT Vue truly novel for its speed and resolution. |
In one case, a winning device's processing power earned it the praise “truly novel” by the judges. RT Vue, an imaging device used by doctors to examine the retina, uses optical coherency tomography (OCT) technology to create microscopic high-resolution cross sections of the retina. It produces detailed images of the retina, down to 5 µm. The device, manufactured by Optovue in Fremont, CA, is used to diagnose retina conditions and diseases such as glaucoma, macular hole degeneration, diabetic retinopathy, and others.
“The 5-µm resolution results from the light source. In OCT, the broader the spectral bandwidth of the light source, the higher the resolution of the image,” says Jay Wei, CEO and president of Optovue. “However, to achieve an image with very high clarity, a special image algorithm is required to remove the noise in the image. We have developed an algorithm that can take advantage of multiple images to be acquired in a short period and can use an averaging method to remove the noise.”
“I was impressed with the RT Vue's ability to capture a high-resolution, three-dimensional profile of the retina in a single pass at speeds never thought possible,” says juror Robert Virag, founder and principal of Trifid Medical Group and Alveoli Medical. “The key to this ability appears to be the advanced software that increases data acquisition speed to 26,000 scans per second—65 times faster than conventional scans.”
Virag says that combined with advanced software noise filters and algorithms that reduce motion artifacts, such processing allows the RT Vue to detect microscopic retinal detachments, atrophy, and microvascularizations that were previously not detectable. “I was also impressed with clarity of the charts and images designed to reduce this complex data to a user-friendly record that is diagnostically useful. Ophthalmologists will undoubtedly appreciate the details more in the future as they see changes in their patient records,” says Virag.
The RT Vue's imaging technologies and its algorithms are at the core of its novelty and functionality. “One feature that distinguishes a digital device such as this is its ability to store images taken over time to monitor the progression of disease or the response to therapy,” says juror Craig Jackson.
Wei says that one of the biggest challenges was leveraging the capability of an advanced technology to solve complex clinical problems and still make the device user friendly in a busy clinical environment. “RT Vue has several unique scan protocols that were designed to acquire and analyze retina and glaucoma diseases,” Wei says. Wei notes that clinicians requested this capability during the clinical investigation stage.
“The scan is performed by a charge-coupled device (CCD) camera and is thus very rapid, with an increased clarity of the image,” says Schneeberger. “It is a leap from the old mechanical method of sampling to a digital one.”
Wiklund says the scan protocols are also an important step forward. “The protocols appear to make it easier for users to proceed efficiently with laser treatment in a clinically appropriate manner,” he says. “The software user interface presents the protocol options in an intuitive and visually pleasing manner on the primary screen, as opposed to making users choose one from a secondary screen. As such, the user interface has a purposeful quality that is not bogged down by introductory information and extraneous options.”
Such technology must also be functional. “Optovue asked us to incorporate its revolutionary OCT technology into a system that was comfortable for the patient and easy for the doctor to use. The company wanted to emphasize its unique qualities of accuracy and speed in a system that was noninvasive and painless,” says Dan Harden, CEO of Whipsaw, an industrial design firm that worked on the RT Vue.
Harden says the controls are easy to learn, so the device immediately fits into a doctor's daily work flow. “Doctors can now offer new clinical insights based on Optovue's extraordinary imaging capabilities. In addition, the device has a wraparound head frame that allows unobstructed communication between doctor and patient.”
The Future Is Wireless
|
Intel's wireless tablet PC was designed specifically to work in medical environments. |
The healthcare industry is demanding clinical settings that are networked wirelessly. One MDEA winner was developed specifically to fill the void in digital patient data processing and networked information systems. The Mobile Clinical Assistant, manufactured by Intel (Beaverton, OR), focuses on the healthcare community's needs to enhance patient safety, reduce medication-dispensing errors, and ease staff work loads.
The device has a 10-in. color liquid-crystal display touch screen; wireless connectivity to access medical records; a Bluetooth wireless stethoscope; a digital camera to enhance patient charting and progress notes; and several patient identity verification technologies, such as radio-frequency identification (RFID) and bar coding, to ensure safe drug administration.
“As we all move toward electronic data entry on patients, devices like the Mobile Clinical Assistant will be used more and more,” notes juror Schneeberger. “I believe that all devices will have to communicate with each other, and with a large company like Intel leading the way, there is hope that a standardized system will be produced,” he says.
It is imperative that such devices blend into the healthcare environment. Robert Jacobs, manager of future platform analysis at Intel, says the team spent a lot of time researching the hospital environments that the device would be used in. “It manifested itself in the kind of peripherals we did—a bar code reader and RFID, for example. It uses technology that is also designed to minimize errors. The patient could be wearing the RFID tag, or the RFID tag could also be used by the nurses if they were wearing a badge,” he says.
Other features were also designed to fit the clinical setting, particularly the fact that it can be wiped down to keep it sanitary. “We knew that keyboards in the medical environment are like petri dishes for bacteria. We were really concerned about the sanitary aspects of it,” says Jacobs.
Jacobs says one of the goals of the device was to use wireless connectivity to make it easier for a nurse to access information. “If nurses want a key piece of data but have to go on a 10-minute fishing expedition to find it, they are likely to decide that it's not important enough to go get it. So we wanted this device to enable them to get to information as quickly as possible,” says Jacobs.
“I'm quite sure that hospitals will become chock full of mobile computing devices in the coming years,” says Wiklund. “Intel's new product impresses on several levels. It appears right-sized for the task of entering and reviewing clinical information, although there is also a role for devices that can fit into a lab coat pocket,” he says.
“It's nice that the device has a large grip and enables stylus-based input, which allows for a greater number of options to be presented on a screen than possible using a touch screen alone,” says Wiklund. “Of course, the value of such a device depends heavily on the interconnectivity of hospital information systems and various stand-alone devices. It also depends on the quality of the software user interfaces developed to manage the flow of information and control of various operations.”
In that sense, says Wiklund, the Mobile Clinical Assistant's fortunes will rise and fall on the quality of the application developed for it. Wiklund points to Intel's report that the device is already having a positive effect on the work flow of phlebotomists who use the device to confirm a patient's identity and ensure that lab work proceeds at the necessary pace.
“Compared with earlier-generation devices that were bulkier and heavier, this one looks lithe and suitable for use by small individuals with short arms that might have trouble comfortably cradling a heavier object,” said Wiklund.
Intel's designers also focused on usability. “We tried to create a systematic way of handling all button presses,” says Jacobs. “The device uses software to standardize the way the platform handles key presses. An applications programmer's interface is included to encourage others to write software to run on the unit.
“The tablet PC with its wireless connections is an incremental advance over the personal digital assistants that were touted a few years ago,” says juror Jackson. “The integration of a stethoscope and bar code reader was good. The real benefits will come from the information being available to the attending physician at the patient's bedside, something probably implicit in the creation of the device.”
Smarter, Safer Systems
Medical devices are becoming increasingly based on software that ensures patient safety and that prevents errors. And, it is now a world in which it is not only feasible, but also expected, that devices will be integrated wirelessly, providing easy access to patient data. These award winners represent the trend toward developing intelligent devices with increased functionality, system integration, and interoperability.
In critical devices, complex software is important to ensuring patient safety. Software helps make the device a smart part of the patient's treatment. Whether it's through wireless connectivity or sophisticated software, the reality is that device manufacturers have a role in increasing patient safety and decreasing medical-related errors.
Copyright ©2007 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like




.png?width=300&auto=webp&quality=80&disable=upscale)
