Customizing Filtration and Separation Materials
Medical Device & Diagnostic Industry MagazineMDDI Article IndexOriginally Published October 2000Medical Plastics & Biomaterials Product developers are using polymers with customization capabilities for a wide range of medical and laboratory applications. Thomas S. Phillips
October 1, 2000
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
Originally Published October 2000
Medical Plastics & Biomaterials
Product developers are using polymers with customization capabilities for a wide range of medical and laboratory applications.
Thomas S. Phillips
In the next few years, there will be a dramatic increase in sophisticated, highly specialized medical and laboratory applications, which will require customized filtration and separation media. Design engineers will be challenged to create more specific and sensitive sampling, testing, and screening products. Part of that challenge will be to keep the products simple, efficient, and cost-effective.
One approach to designing filtration or separation products for new applications is to add on to an existing design. Many designers tend to add prefilters or multilayers of filtration media to existing materials designs. The resulting products can exhibit the standard vices of complex assemblies: increased manufacturing and assembly times, long changeover times in the field, and an increase in the number of materials held in inventory. These conditions can raise costs for the manufacturer and user alike.

Porous UHMW polyethylene is used in pleated laminates for filtration (left), HPLC columns (bottom), and filtration baskets for laboratory sampling (right).
How can a product developer reduce complexity and still achieve the end goal of a specialized filtration application? By forming a partnership, a designer and materials supplier can work together to simplify the end-product design and reduce manufacturing and assembly times and costs while enhancing filtration performance. Collaboration can also reduce the product development cycle time and, consequently, time to market. The overall result is a customized media tailored to the end-customer's needs within a specified time frame.
NEW CHALLENGES
For a number of reasons, the medical and laboratory product industries cannot rely solely on the current forms of filtration and separation materials for new applications.
Chemical Environment. Some filtration and separation processes involve the use of strong solvents, reagents, or corrosive chemicals. Many existing nonpolymeric materials, such as nitrocellulose and paper, cannot function adequately in these chemically harsh environments. In addition, the physical integrity of the material must be maintained in these applications.
Analyte Detection Limit. Many laboratory applications use analytes in the parts-per-million or parts-per-billion range. Contaminants adhering to or adsorbed by the filtration medium can interfere with the analysis. A chemically inert and pure medium is best for processing such samples.

Cross section of porous UHMW polyethylene, with 25-mm-diam pores, shows tortuous paths in the material.
Specificity. Biotechnology analyses such as those used in medical and pharmaceutical laboratories are involving extremely specialized testing to a greater extent. Incorporation of specific functional groups in a separation media is highly desirable. This is particularly evident in diagnostic assays and in separations using high-performance liquid chromatography (HPLC).

Chemical extraction bags are created by die-stamping a laminate of porous UHMW polyethylene and ePTFE.
In addressing these challenges, suppliers have developed new polymeric filtration and separation media that are durable and chemically pure, and provide the physical stability necessary to withstand demanding process conditions. Porous polymers, in particular, lend themselves to customization. In addition, there are many treatments available that can chemically alter polymeric filtration materials to meet the requirements of the final application.
ULTRAHIGH-MOLECULAR-WEIGHT POLYETHYLENE
Because porous ultrahigh-molecular-weight (UHMW) polyethylene is an FDA-compliant material that is already used in a wide range of medical and laboratory filtration and separation applications, it provides a good example of the customizing process. UHMW polyethylene is a chemically resistant, long-chain polymer of ethylene with an extremely high molecular weight—3.1 million amu or above. Because of its high molecular weight, the polymer maintains abrasion resistance and strength even when it is made porous for filtration or separation applications. The porous form, which can be pleated, is easily handled in manufacturing. It can be precision skived into films as thin as 0.002 in. And because no processing additives are used during manufacturing, the chemical purity of the material is maintained.

A specially designed blade moves into a turning billet of porous UHMW polyethylene, skiving off the film.
The porosity and pore size of UHMW polyethylene can be adjusted per the end-users' requirements. The porosity is uniform in all three axes, which can be vital to constant liquid flow in filtration and separation. The tortuous path of the pores through the medium—much like that of a sea sponge—makes porous UHMW polyethylene an efficient filtration medium and allows for flow control by customizing the porosity. In its naturally hydrophobic state, porous UHMW polyethylene is an excellent medium for filtering solutions containing laboratory solvents such as alcohols, ketones, aromatics, and strong organic acids or bases. For separations involving aqueous solutions or biological fluids such as blood, the material can easily be converted to a hydrophilic form.
WORKING WITH MATERIALS SUPPLIERS
Collaborating with an experienced supplier is a straightforward process. The supplier needs to bring a certain level of expertise to the general development process to accomplish customization. There are a number of attributes that a filtration or separation product developer should look for in a materials supplier.
Market Experience. Selecting a materials supplier who has past experience customizing materials for the medical and laboratory markets is more than simply reassuring to the product developer. It means that the supplier understands the rigorous demands of medical product development and has a depth of expertise in developing materials for similar applications.
Library of Customized Materials. A materials supplier who has previously created a wide range of customized materials can considerably shorten the engineering process. Even if no appropriate porous medium exists that meets the specifications for the new application, the supplier often will have developed materials with similar porosity, flow rates, and pore sizes that can be used as a starting point for customization.
Library of Resins. An experienced materials supplier is familiar with the capabilities of readily available, single and combination resins. Particle size, molecular weight, and density of the resin directly affect the nature of the customized material.
Library of Surfactants. By adhering to the substrate, surfactants coat the surface of a material. Some surfactants can counteract the hydrophobic properties of UHMW polyethylene, making the membrane hydrophilic. Others may create an affinity for specific chemicals, allowing them to selectively adsorb one chemical species over another.
Library of Plasma Treatments. Unlike the use of surfactants, the plasma treatment process does not use additives that adhere to the substrate, so it leaves virtually no extractables behind. Plasma treatments expose the substrate to an ionized gas. Different gases, such as nitrogen, hydrogen, or ammonium, impart different properties to the substrate. For these reasons, using a filter membrane customized with a plasma treatment can be an attractive choice for a product that must filter very low concentrations of analytes, such as drugs. UHMW polyethylene is easily customized using this technique.
Relationships with Outsourcers. Confirming the specifications for a material may require testing or processing that neither the materials supplier nor the product developer can perform on-site. For example, in some air filtration applications, it might be important to know the moisture vapor transmission rate of a sample of UHMW polyethylene that has been treated with a surfactant. Under these circumstances, the supplier should arrange for a specialized off-site laboratory to perform the tests. Off-site testing should be performed in accordance with any required certifications, such as ISO or ASTM. Also, a materials supplier's established relationship with converters—e.g., a die-cutter in the case of HPLC frits—can help to deliver finished parts, simplifying product development.
Product Traceability. Traceability for customized polymers should extend from the initial resin batch to the shipped final product. A supplier for the medical industry should meet ISO 9001 or GMP requirements and should be able to establish the quality of each batch of materials provided for use in the medical or laboratory product. Traceability is vital for quality assurance, which in turn promotes repeatable results when using the same filter medium. If the materials supplier has a rigorous tracking system already in place, that will also accelerate the design process by allowing the product developer and the materials supplier to employ a preestablished product documentation path.
STEPS IN THE CUSTOMIZING PROCESS
Sample Development. Initially, the product developer should provide the supplier with specifications for the filtration or sampling application, rather than offering the specifications of the material previously in use for that application. Establishing flow rate and volume, pressure, use temperature, pore size, and chemical environment is more useful than, for example, determining the density of a previously used substrate.
Also important are the specifications for manufacturability: Is the material subject to any heat, stress, or tension during manufacturing or processing? Frequently, the manufacturing process is a more rigorous environment for the material than the final application. For instance, in the manufacture of wicking products, the materials-handling system can put tension on narrow strips of wicking material that can snap if the wicking material is inelastic. The resulting scrap increases overall production costs. UHMW polyethylene can be customized to combine elasticity with high tensile strength, creating a product that can withstand the materials-handling forces.
Once the overall specifications for the material have been determined, the materials supplier can choose a customizing method that will create the best fit for the application. If the requirements cannot be met by a single filter membrane, a laminate of UHMW polyethylene and another material can be considered. Treatment with a given surfactant may provide a sufficiently hydrophilic surface for the substrate, or the substrate may require plasma treatment to prevent the introduction of potential extractables that might result from a surfactant treatment.
Creation of a special resin material may be the best option. For example, activated carbon is a common medium for air filtration. Activated carbon is often used in a granular form or is supplied as a woven cloth for layering with another substrate. However, a materials supplier can imbed the activated carbon directly in UHMW polyethylene during manufacture. The resulting porous UHMW polyethylene material contains the activated carbon locked within the structure of the membrane. Such an application permits adequate airflow and does not interfere with the adsorptive properties of the activated carbon.

Porous UHMW polyethylene can be installed in the end of HPLC columns for laboratory applications.
Carbon is not the only potential additive. The possibilities for incorporating different materials into porous UHMW polyethylene are limited only by the temperature stability of the materials, since porous UHMW polyethylene billets are sintered at roughly 300°F.
Testing, Customer Feedback, and Revision. The materials supplier will test each sample, employing off-site labs for special tests. Customer field-testing and evaluation follow. All this testing serves to fine-tune the material's specifications.
In-house, the supplier would test the material for pore size and uniformity, porosity, water entry pressure, airflow rate, and tensile and dielectric properties. Each materials lot would be tested individually and the results reviewed for necessary revisions to the customized material.
For example, after seeing the test results of an initial sample of UHMW polyethylene in a wicking application, a product designer might request that the material be made more hydrophilic to promote a faster rate of capillary flow. For many applications, this can be accomplished simply by applying a different surfactant to the UHMW polyethylene. It is during this fine-tuning process that the materials supplier's past experience and library of customizing techniques are of the most value.
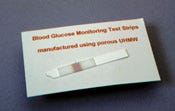
Because it can be readily converted to a hydrophilic form, UHMW polyethylene is well suited for wicking applications.
Final Testing. Normally the product developer is responsible for field-testing the customized material in the finished product, since laboratory and medical product developers follow strict in-plant quality programs. It is still possible to customize the UHMW polyethylene at this point; however, the previous tests should already have refined the material until it is the best possible fit for the application.
Once the product developer has accepted the material, documented testing of each lot continues when the material is in production. The entire customizing cycle usually requires one to three months.
CONCLUSION
Using customized materials in medical and laboratory products can reduce manufacturing and assembly costs, as well as improve the performance of the final product. Partnering with the materials supplier ensures incorporation of key product performance requirements into the initial design, allowing the product designer to meet tighter specifications for the overall product. Also, filtration materials customized for best fit and performance promote product consistency.
Familiarity with new materials on the part of a product developer can lead to the development of entirely new products. For example, the light-absorbing properties of porous UHMW polyethylene are presently being investigated for use in infrared technology applications.
Customized polymers have gained wide acceptance in medical and laboratory applications, but full exploitation of their potential has barely begun. The use of multimaterial laminates as substitutes for thick-layered filter membranes is still largely unexplored. Before the advent of customized polymers, available filtration and separation technology left product developers asking themselves, "Can we do this application at all?" Now the pertinent question has become, "What's the best way to do it?"
Thomas S. Phillips is product development manager for porous, conductive, bulk, and surface-modified products at DeWal Industries Inc. (Saunderstown, RI).
Back to the MDDI October table of contents | Back to the MDDI home page
Copyright ©2000 Medical Device & Diagnostic Industry
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
