A New Design for Opioid Overdose Treatment
An industrial designer witnessing opioid addiction in his community creates a new, improved device designed to deliver treatment in case of an overdose.
June 2, 2016
An industrial designer witnessing opioid addiction in his community creates a new, improved device designed to deliver treatment in case of an overdose.
News this week that Prince's death was a result of opioid overdose has brought more attention to America's opioid crisis. If you saw a friend or stranger overdosing on heroin or another opioid, would you know what to do? If you had the treatment for overdose, a medication called naloxone, in hand, would you be able to administer it in time?
Jonathan Grossman, an industrial designer at Frog Design (frog) in San Francisco, was already aware of the opioid addiction crisis and its effects on the community and people he walks by everyday in the city. But it wasn't until he watched an online documentary about the opioid epidemic and learned that a naloxone syringe is one of the best weapons against overdose that he realized his skills could help improve care for people dependent on opioids.
Grossman explained that while viewing the documentary, "I'm watching them assemble [the naloxone syringe] and I'm saying, 'Oh my gosh, this is a terribly designed syringe.'"
Get inspired to innovate in medtech at the MD&M East Conference, June 14-16, in New York City. |
Delivery methods for naloxone include a glass vial and syringe, a nasal atomizer, and an auto-injector. The auto-injector, Evzio, was given priority review and approved by FDA in April 2014. (Evzio was a bronze 2015 MDEA winner in the Drug-Delivery Devices and Combination Products category.) At several hundred dollars, Evzio is expensive if being purchased by nonprofits, though maker Kaléo does make product donations. A naloxone nasal spray, Narcan, was approved by FDA in November 2015. Narcan was the first FDA-approved nasal spray, though kits that use an atomizer to deliver the injectable version of the drug were already common. These nasal atomizer kits are widely used, but Grossman pointed out that the current design requires nonprofits to assemble the devices, order various quantities of different components, manage those inventories, and ensure each device is put together correctly.
Attention around opioid addiction is increasing. In March, the Obama administration announced its plans to battle the opioid crisis, including $11 million in funding to the states to buy, distribute, and train people to administer naloxone and other overdose preventive treatments. Just a week ago, FDA approved a buprenorphine implant for treatment of opioid dependence.
"Opioid abuse and addiction have taken a devastating toll on American families. We must do everything we can to make new, innovative treatment options available that can help patient regain control over their lives," FDA commissioner Robert Califf, MD, said in the FDA release announcing approval of the buprenorphine implant.
So while there are overdose prevention options available, there is still plenty of room for new offerings and ideas.
|
Grossman |
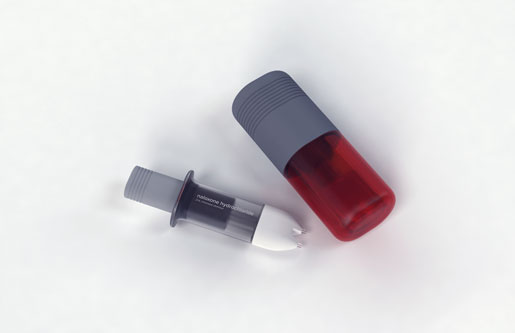
Grossman ticked off the pain points with the design of the nasal atomizer kits commonly used by nonprofits: "Every time you open the box you have to remove four different caps from different parts of the body. There's the nasal atomizer syringe--when you twist it on, it's actually really hard to get it aligned properly and if it's misaligned, naloxone will spray out the side of the syringe when you depress it. There are issues associated with assembling a syringe in an emergency situation. It's just not an ideal experience." He saw an opportunity to conceptualize a new design.
The design needed to fit the user environment, said Lindsey Mosby, executive strategy director and healthcare practice lead at frog. "We're talking about people who are scared, who are nervous."
Grossman began working on designs in his spare time. He explained that after identifying the current pain points, he creates a list of design principles to help him ideate designs, asking himself how a new design could reduce assembly and make it easier to administer a single dose. "You can think very specifically about each design issue and then you start to synthesize these smaller concepts into bigger concepts," he said.
It was important to make the design "robust" enough to handle bouncing around in a backpack, Grossman said. "You'll notice we used heavier plastics and thicker forms that speak to this robust nature so that people won't be worried that it will accidentally administer itself in your bag."
Grossman sketched out a few design concepts for a nasal atomizer, including a pre-filled syringe that would make it clear when the user needed to switch to the other nostril, a dual-nostril atomizer with a tube-like look, and a dual-nostril atomizer with a pill-like shape that split the single dose for each nostril.
It was the third design that frog believe "had the right balance of robustness and accessibility," according to a document summarizing the design process. The device has an open, clear package with a grippable cap that is opened to reveal the syringe plunger. The dual-nostril, clear body design means users don't have to switch nostrils and can see that the naloxone is being dispensed. A soft foam around the nostrils should make it a fit for all noses as well.
While there's a great need for opioid overdose treatment, Grossman pointed out that the device design could have a place in other applications, including administration of endotracheal drugs in an emergency room setting.
The atomizer started as a concept project for Grossman in his spare time, but frog is now looking for a partner to move forward with the design.
Mosby explained that "passion projects" are regular undertaking at frog. "In a field like healthcare where it's hard to innovate . . . most of our clients are not in a position to go white space. If we can do some of that work on 'frog time,' but then put that out there and have a potential partner come forward . . . anything that might bring something to market sooner rather than later--that's a great thing."
[Images courtesy of FROG DESIGN]
About the Author(s)
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)
