Subcutaneous Drug Implant
November 10, 2014
Subcutaneous Tuberculosis Drug Implant |
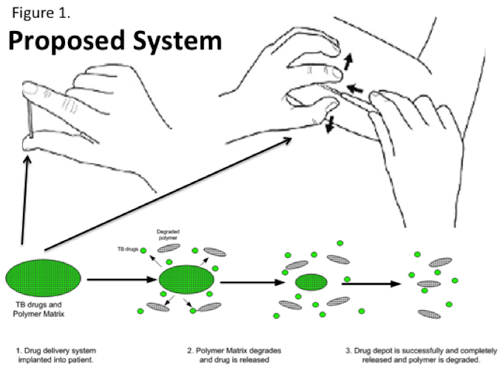
How does the device work?The device is a subcutaneous drug implant for tuberculosis medication for an entire therapeutic course. By leveraging the technology of other implantable drug delivery systems (e.g., Norplant), this device would contain an entire 6-month treatment regime (one-time therapy) for tuberculosis. After the treatment course is completed, the device would be biodegradable. What problem in healthcare does it solve?Tuberculosis is the 2nd leading infectious disease killer, after HIV, in the world. There have been an increase in TB expenditures, from $500M to 1$1.8B over last 10 years. Current therapies require a 6 month total regimen, but face significant challenges of patient adherence because the treatment is burdensome, requires frequent visits, and symptoms improvement predates cure. This becomes a huge public health problem as the tuberculosis then develops multi drug resistance, passing to other members of the community and becoming ever harder to treat. Why should the device be commercialized?Tuberculosis is a huge issue in global health. There were 8.8M new cases in 2010, and 1.4M deaths. A significant burden of global tuberculosis is multidrug resistant tuberculosis (MDR -TB), with 440k new cases and 140k deaths annually. Commercialization of this device would ensure that a single intervention -- implantation of this device -- would ensure a cure for patients while eliminating risk of disease transmission. What inspired you to design this device?I have long believed that surgery and global health have a huge overlap. Though the old paradigm of global health was related solely to infectious diseases (malaria, HIV, TB), newer data shows that surgery can be cost-effective and high-yield in many settings. This intervention is a marriage of both fields -- a surgical intervention that can have lasting public health impact.   |
You May Also Like