Could an All-Plastic Design Make TAVR 30 Times Safer?
September 14, 2015
Direct Flow Medical explores the next generation of transcatheter aortic valve replacement devices made from polymers and completely free of metals. The company says that the all-plastic design could result in a safety profile that is a quantum leap better than that of TAVR valves with metal-based frames.
Kristopher Sturgis
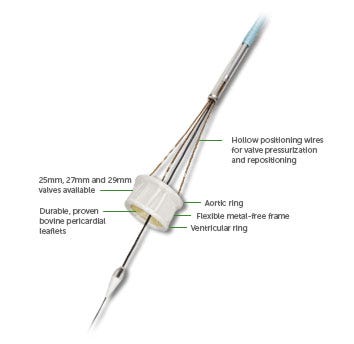 While catheter-based heart valves represent a much-less invasive treatment option than traditional surgical valve replacement, the procedure is not without its risks. A significant number of patients receiving TAVR devices face problems with aortic regurgitation (AR), which can lead to more-serious complications, says Randolph Chitwood, MD, director of surgeon relations for Direct Flow Medical.
While catheter-based heart valves represent a much-less invasive treatment option than traditional surgical valve replacement, the procedure is not without its risks. A significant number of patients receiving TAVR devices face problems with aortic regurgitation (AR), which can lead to more-serious complications, says Randolph Chitwood, MD, director of surgeon relations for Direct Flow Medical.
Based in Santa Rosa, CA, where Medtronic also has its vascular headquarters, Direct Flow Medical is developing an all-plastic TAVR device it says could minimize the risk of AR. "From the surgical experience, it was very clear that AR was bad for patients," Chitwood says. "We wanted to build a device that left the physician in control of the procedure, having the ability to fine tune the position of the device, and assess the result prior to committing to implantation. We wanted the final implant to be like the gold standard, a surgical valve."
Ultimately, the solution came in the form of an inflatable device with a formed-in-place support structure. This completely new approach enables any surgeon to implant the device and specifically position the valve, allowing for it to be precisely placed within the heart. All of this is made possible through the complete substitution of all metal materials with new polymer-based materials.
Chitwood will be speaking about the new device and the benefits of using polymer materials at MD&M Philadelphia, which runs October 7 to 8. In the following Q&A, Chitwood elaborates on Direct Flow Medical's new approach to transcatheter aortic valve replacement devices, and how their new polymer-based device could serve as the new gold standard in valve technology, and improve treatment for patients whose aortic valve opening has narrowed.
MPMN: What went into the process of selecting the right polymer for this device, and what sort of advantages does it offer?
Chitwood: We needed something that in a liquid form had very low viscosity, solidified quickly, was radiopaque, and was tolerable for patients if it was inadvertently spilled into the bloodstream. In the solid form, it had to form the permanent support structure of the device. It had have high strength, be creep resistant, biocompatible, etc. We conducted an exhaustive search on available platforms and found nothing that adequately met our needs. So we had to develop it ourselves.
We started from the ground zero. We built a polymer chemistry lab and put together a team of device development engineers, material scientists, and PhD polymer chemists. We looked at countless different chemical families and settled on a water soluble epoxy backbone, with an organically-bound iodine additive for radiopacity. The real discovery came in finding the right amine blend. This is where, through a lot of experimentation, we were able to develop something that met all those divergent characteristics.
|
Plastic planes an integral role in the design of the entire TAVR device, including in the delivery system. |
MPMN: What were some of the biggest challenges you faced in developing this device, specifically from a materials standpoint?
Chitwood: Developing the polymer was a big challenge, but it actually solved a lot of other issues and presents quite a few opportunities. Stent frames, especially nitinol frames, are by necessity designed to be right on the edge of failure. With our polymer frame, we have a safety factor of more than 30 times that of metal-based frames. Having a polyester covered, balloon inflatable frame allows us the ability of not having to deform our support structure in order for it to fit into a low profile catheter for delivery. Because we don't have to accommodate for additional room to deliver our support structure, we can utilize the same valve thickness and material as surgical valves. Our bovine tissue is the same thickness and processed the same ways as surgical valves. These features will become very important as TAVR therapy moves from extreme risk to lower risk AS patients. ...
We believe we created a better transcatheter valve platform. We believe this technology platform has the potential to be revolutionary. This is the first and only commercial iteration of a polymer TAVR device and we are planning on further development and future iterations. It was designed to be a very versatile platform. European physicians have already put our device (off-label) in various other valve positions to treat tricuspid regurgitation and mitral regurgitation. We believe there are clear advantages to this technology in the mitral position and we are developing a platform and clinical testing program for this indication.
MPMN: Aside from the material differences, what would you say sets this Direct Flow device apart from those made by other companies including Edwards LifeSciences and Medtronic?
Chitwood: Well I think what defines Direct Flow for me is the clinical results. The bottom line, it's about the patient. When we talk about things like survival and simple measures of quality of life in these extreme risk patients--where the Direct Flow Medical valve has been studied--we really stands apart from the field. We believe our Discover [troa;] 2-year data demonstrates best in class survival and AR reductions when compared to data from trials in similar populations.
The DFM device also offers some other advantages for the implanting physician, being in control during the procedure. That fact that our platform is immediately functional upon inflation allows the physician the ability to take their time in delivering the device to its appropriate position. Our platform is repositionable with millimetric control for fine tuning. Our platform also conforms to the patient's anatomy. As we all know, no two patients are exactly alike. By precisely conforming to the patient's anatomy we can virtually eliminate aortic regurgitation. And aortic regurgitation which has been shown to be associated with increased survival rates. Our platform is also designed to be fully retrievable. This allows the physician the ability to change device sizes if needed or the ability to leave nothing behind if the desired result isn't obtained.
MPMN: What sort of insights can you share about early clinical trial results?
Chitwood: Our U.S. pivotal trial Salus is in progress and currently enrolling patients. Our European CE mark Discover trial 2-year data showed excellent results with 80% survival, 100% mild or less aortic regurgitation, with 93% of the patients being in NYHA Class I or II in these extreme risk patients. We also demonstrated sustained hemodynamics in EOA and valve gradients through 2 years. We also have postmarket registries that show very similar results: Naber.... Discover Registry....
MPMN: How soon do you expect these new TAVR devices to hit the market? Are there any significant challenges that need to be addressed before that time arrives?
Chitwood: We launched commercially in Europe in 2013 and are closing in on 2000 procedures. We started the U.S. Salus Pivotal trial in Q4 of 2014 and will need to enroll nearly 1000 patients in 45 sites in a 2:1 randomized fashion. Post-trial enrollment we will need 1 year follow up on these patients and then filing with the FDA. It's still too early to speculate on a date for U.S. commercial lunch.
(Chitwood will be speaking about the new device and the benefits of using polymer materials at MD&M Philadelphia, which runs October 7 to 8.)
Kristopher Sturgis is a contributor to Qmed and MPMN.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like



