Quality Management for Cleanroom Operations
Medical Device & Diagnostic Industry Magazine MDDI Article IndexAn MD&DI February 1999 ColumnCLEANROOMSEfficient contamination control requires a system that applies total quality management principles.
February 1, 1999
Medical Device & Diagnostic Industry Magazine
MDDI Article Index
An MD&DI February 1999 Column
CLEANROOMS
Efficient contamination control requires a system that applies total quality management principles.
Designing medical devices, building controlled environments, and submitting devices for FDA approval are time-consuming, costly activities for medical device manufacturers. Such activities generally have a defined time line with a set completion date and budget. However, the day-to-day operations of manufacturing in a controlled environment present continual challenges that vary as regulations change and the cost of manufacturing increases. This article presents a system designed to help ensure efficient contamination control operations.
THE PACT SYSTEM
The PACT—prevention, assessment, corrective action, and training—system (Figure 1) is designed to assist supervisors, managers, and engineers with contamination control management. It embraces the continuous improvement principles of total quality management.
 Training and proper gowning are essential preventive activities. Photo courtesy of Burron OEM Division of B. Braun Medical Inc. (Bethlehem, PA).
Training and proper gowning are essential preventive activities. Photo courtesy of Burron OEM Division of B. Braun Medical Inc. (Bethlehem, PA).
Prevention. Personnel training, proper gowning, and housekeeping maintenance are essential preventive activities for controlled environments. Manufacturers have known for decades that people are a major source of contamination. Therefore, training employees and providing appropriate garments are critical tools for controlling contamination caused by humans. Workers' normal activities—for example, rapid arm motions or fast walking—can compound contamination because these actions are difficult to control and impossible to police. Such seemingly innocuous activities become a serious issue in the proximity of the process and product.
Human-generated contamination can be isolated by a body filter that creates a barrier. Cleanroom garments are designed to provide this barrier and reduce the inherent contamination from human debris such as hair, dandruff, clothing lint, coughing, skin flakes, dust particles, skin oils, and so on. Although the cleanroom's particulate and microbial control levels govern the minimum gowning requirements, the product and process may require additional personnel gowning protection. The Institute of Environmental Sciences and Technology provides guidance on garment selection, specifications, and testing.1
The cost of a garment system can be a significant portion of the total operational budget. Reducing the laundry cycle, rewearing hairnets and shoe covers, or eliminating gloves are not viable approaches to operational cost control. Such shortcuts will result in increased foreign matter, increased contamination from skin and oils, increased bioburden, and increased housekeeping maintenance. Ultimately, they can lead to product reworks or rejects. A risk assessment must be performed to address any cost-savings program that could affect product quality or reliability.
 Prevention, assessment, corrective action, and training help ensure efficient cleanroom operations (Rexam; Matthews, NC).
Prevention, assessment, corrective action, and training help ensure efficient cleanroom operations (Rexam; Matthews, NC).
Assessment. Routine monitoring, internal auditing, and third-party auditing are essential ingredients in any quality program, including cleanroom operations. Monitoring of air, surfaces, and personnel provides the data required to evaluate compliance and review environmental control. Routine particle counting should be performed at least weekly, with critical operations monitored daily. Although monitoring at 0.5 µm is the industry standard, additional monitoring at 5 µm and larger offers additional information about product quality and exposure in the controlled environment.
Surface monitoring should be performed weekly to determine whether housekeeping measures are adequate. Visual inspections can be used to assess the frequency of general housekeeping maintenance activities, including wiping, mopping, and sanitizing. This inspection can be limited to a wipe test but can also include ultraviolet or high-intensity, oblique white-light inspection techniques.2
Microbial monitoring of air and surfaces should also be performed routinely, with critical areas monitored daily. (Noncritical areas can be monitored weekly.) Contact plates or swabs provide an effective means of monitoring surfaces. Air sampling can be performed using a variety of methods, including impacters—slit, sieve, and centrifugal—impingers, and settling plates.
A multidisciplinary team should perform internal auditing. In general, the team should use checklists, specification requirements, and other methods to assess compliance and should note observations and discuss them with appropriate managers. Compliance issues must be identified so that managers can determine both corrective and preventive actions for each issue. The team should also ensure that the identified compliance issues are tracked to their final resolution. For example, internal audit findings could be assigned to a department or an individual for final resolution. The auditing team would subsequently review all responses, verify that the corrective actions are satisfactory, and confirm completion.
An external audit provides a different perspective and often takes a different approach than an internal audit. For example, because external auditors work with other device manufacturers, they have a basis of comparison that internal auditors cannot provide. External auditing and assessment should be performed annually. Benchmarking the results can provide not only the ranking of achievements, but can also identify areas in which further improvement is necessary.
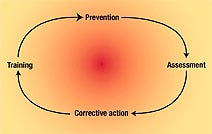 Figure 1. The PACT system of quality management provides a method for tracking cleanroom operations.
Figure 1. The PACT system of quality management provides a method for tracking cleanroom operations.
Corrective Action. When an out-of-specification issue is identified, a corrective action program, resolution deadline, and preventive plan should be implemented. For every issue, the root cause should be identified and appropriate measures established to prevent recurrence. This ensures that the underlying problem is addressed. Implementing a quick fix usually addresses only the symptoms. Quantitative reports on the causes and corrective actions provide a tool to track the corrective action plan and evaluate the effectiveness of preventive measures.
Annual Training Session. A thorough training session is critical for establishing and maintaining efficient controlled-environment operations. An annual training program must include the quality system regulation, proper hygiene, basic microbiology, and contamination control. Employees cannot work effectively in controlled environments without a clear understanding of the science behind contamination control. Discussions of contamination control principles should address gowning, disciplines, restricted items, prohibited personnel actions, and other measures to minimize contamination. Classroom instruction followed by hands-on training in gowning and workstation procedures works best for training adults. Additional training or retraining needs may result from subsequent assessments or corrective action plans.
CONCLUSION
It is imperative that contamination control operations run efficiently to ensure product quality. Total quality management principles do apply to contamination control. Prevention, assessment, corrective action, and training are essential to a successful operation.
A certification program is in the future for all controlled-environment personnel. This type of program would include competency testing for all levels of worker performance. Testing would cover not only contamination control issues, but also product procedures, product specifications, equipment and manufacturing specifications, and regulatory requirements.
REFERENCES
1. IES Recommended Practice 3.2: Garment System Considerations for Cleanrooms and Other Controlled Environments (Mt. Prospect, IL: Institute of Environmental Sciences and Technology, 1993).
2. IES Recommended Practice 18.2: Cleanroom Housekeeping—Operating and Monitoring Procedures (Mt. Prospect, IL: Institute of Environmental Sciences and Technology, 1992).
Anne Marie Dixon is managing partner of Cleanroom Management Associates (Carson City, NV).
Copyright ©1999 Medical Device & Diagnostic Industry
You May Also Like


