Outsourcing Outlook on Cleanroom Manufacturing and Assembly: Products
October 4, 2013
Molding of critical-care devices Aberdeen Technologies Inc. performs insert molding, micromolding, and liquid-silicone-rubber molding operations in a Class 100,000 GMP-compliant cleanroom. Additional manufacturing capabilities include tube slitting and skiving, bonding, assembly, marking, leak testing, and heat-sealing. The company also provides services to manufacturers with devices under development, offering product and process design, development assistance, and full-scale in-house mold tooling construction. It employs programmable indexed rotary tables that can minimize cycle times and allow for secondary operations to be carried out at the molding machine. The company has produced molded components and subassemblies for such critical-care applications as implantable microdevices, light-refraction oximeters, and biosensors.
Aberdeen Technologies Inc. performs insert molding, micromolding, and liquid-silicone-rubber molding operations in a Class 100,000 GMP-compliant cleanroom. Additional manufacturing capabilities include tube slitting and skiving, bonding, assembly, marking, leak testing, and heat-sealing. The company also provides services to manufacturers with devices under development, offering product and process design, development assistance, and full-scale in-house mold tooling construction. It employs programmable indexed rotary tables that can minimize cycle times and allow for secondary operations to be carried out at the molding machine. The company has produced molded components and subassemblies for such critical-care applications as implantable microdevices, light-refraction oximeters, and biosensors.
Aberdeen Technologies Inc.
CAROL STREAM, IL
Cleanroom thermoformed packaging Maintaining Class 10,000 and Class 100,000 cleanroom manufacturing space certified to ISO 13485:2003 and ISO 9001:2008 standards, T.O. Plastics manufactures custom thermoformed packaging for medical devices. Specializing in trays made from PETG and polycarbonate, the company has capabilities in thin- and heavy-gauge thermoforming, maintaining a facility that is equipped with a high-speed in-line thermoforming system. It also offers in-house design, rapid prototyping, and tooling services. The vendor provides 100% product inspection, HEPA and ionization-based air cleaning, and burr-free trimming using heated forged dies, line-rule dies, or matched metal dies.
Maintaining Class 10,000 and Class 100,000 cleanroom manufacturing space certified to ISO 13485:2003 and ISO 9001:2008 standards, T.O. Plastics manufactures custom thermoformed packaging for medical devices. Specializing in trays made from PETG and polycarbonate, the company has capabilities in thin- and heavy-gauge thermoforming, maintaining a facility that is equipped with a high-speed in-line thermoforming system. It also offers in-house design, rapid prototyping, and tooling services. The vendor provides 100% product inspection, HEPA and ionization-based air cleaning, and burr-free trimming using heated forged dies, line-rule dies, or matched metal dies.
T.O. Plastics
CLEARWATER, MN
Medical electronic assemblies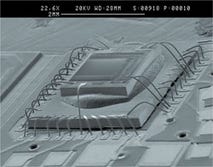 Valtronic Technologies, an engineering and contract manufacturing company, concentrates on the manufacture of electronic assemblies for medical devices ranging from cochlear implants to neurostimulators and heart-monitoring equipment. Operating ISO Class 7 and Class 8 cleanrooms, the company can handle small preproduction runs or large-scale full-production volumes. It is FDA inspected, and its quality-management systems are certified to ISO 13485 and ISO 9001 standards. Cleanroom capabilities include gold-ball and aluminum wire bonding; silicon encapsulation; and glued flip-chip assembly using automatic, manual, and pick-and-place machines.
Valtronic Technologies, an engineering and contract manufacturing company, concentrates on the manufacture of electronic assemblies for medical devices ranging from cochlear implants to neurostimulators and heart-monitoring equipment. Operating ISO Class 7 and Class 8 cleanrooms, the company can handle small preproduction runs or large-scale full-production volumes. It is FDA inspected, and its quality-management systems are certified to ISO 13485 and ISO 9001 standards. Cleanroom capabilities include gold-ball and aluminum wire bonding; silicon encapsulation; and glued flip-chip assembly using automatic, manual, and pick-and-place machines.
Valtronic Technologies (USA) Inc.
SOLON, OH
Imaging, orthopedic, and surgical components Located in a 50,000-sq-ft facility, 3D Medical Manufacturing Inc. provides the medical device industry with complete cleanroom manufacturing services. The contract manufacturer uses its cleanroom to fabricate and assemble components and products such as lens devices for fluoroscopy and endoscopy equipment, orthopedic implant parts and instruments, radiology equipment, and general surgery devices. With more than 1600 sq ft of space designated in its facility for cleanroom and assembly activities, the company specializes in a range of capabilities that include prototyping, packaging, and logistics. The FDA-compliant manufacturing facility is certified to ISO 13485:2003 and ISO 14644-1 standards.
Located in a 50,000-sq-ft facility, 3D Medical Manufacturing Inc. provides the medical device industry with complete cleanroom manufacturing services. The contract manufacturer uses its cleanroom to fabricate and assemble components and products such as lens devices for fluoroscopy and endoscopy equipment, orthopedic implant parts and instruments, radiology equipment, and general surgery devices. With more than 1600 sq ft of space designated in its facility for cleanroom and assembly activities, the company specializes in a range of capabilities that include prototyping, packaging, and logistics. The FDA-compliant manufacturing facility is certified to ISO 13485:2003 and ISO 14644-1 standards.
3D Medical Manufacturing Inc.
Riviera Beach, FL
Wound-care-device production Boyd Technologies LLC, a company specializing in the converting and contract manufacturing of medical disposables, offers expanded capabilities in wound-care device production. Working in a variety of controlled environments ranging from modular cleanrooms to equipment-isolation structures, the company produces components and finished products from technical papers, nonwovens, membranes, films, foils, extruded netting, and nanofiber materials. Formerly a manufacturer of consumer bandages and wound-care products, the company has continually upgraded its validation procedures and GMP levels so that it can meet medical device industry requirements. A new Class 7 cleanroom dedicated to wound-care production will add to its medical device fabrication capabilities.
Boyd Technologies LLC, a company specializing in the converting and contract manufacturing of medical disposables, offers expanded capabilities in wound-care device production. Working in a variety of controlled environments ranging from modular cleanrooms to equipment-isolation structures, the company produces components and finished products from technical papers, nonwovens, membranes, films, foils, extruded netting, and nanofiber materials. Formerly a manufacturer of consumer bandages and wound-care products, the company has continually upgraded its validation procedures and GMP levels so that it can meet medical device industry requirements. A new Class 7 cleanroom dedicated to wound-care production will add to its medical device fabrication capabilities.
Boyd Technologies LLC
SOUTH LEE, MA
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
