MD&M East Supplier Focus: CRI
June 10, 2015
|
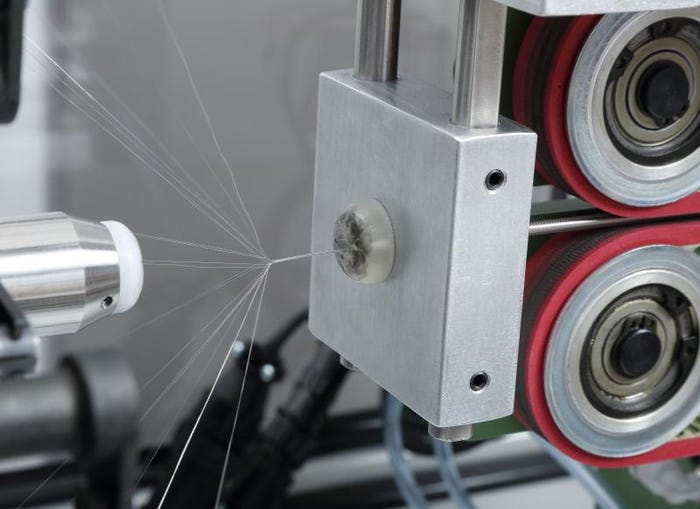
Braided catheter manufacturing shown in CRI's facilities. |
CRI (Booth #549; Indiana, IN) has expanded their product and service offerings to include interventional products.
Brian Buntz
"In addition to our new interventional product line, we continue to provide full product life cycle management including research, design, development, prototyping, verification and validation, testing, regulatory, mass production and distribution," says Renée Clinard, the company's sales manager.
In the following Q&A, Clinard discusses this technology in more depth and reflects on broader trends in the medical device industry.
Qmed: What is the best thing about this technology or service, and what kind of innovations could this technology enable?
Clinard: All facets of our medical device and catheter development have grown through utilization of our in-house capabilities that consist of laser welding, balloon bonding, balloon blow molding, polymer extruded shaft capabilities, braided shafts, and coiled shafts.
|
A CRI balloon catheter at the company's booth at MD&M East. |
Qmed: How do you see the demands of medical device manufacturers changing in 2015?
Clinard: The aging generation of Baby Boomers, growing health concerns, and strict healthcare regulations are pushing medical device manufacturers to expand operations to meet heightened demand. Manufacturing organizations are investing in next-gen ERP solutions incorporating innovative analytics that enhance productivity and reduce operational costs.
Qmed: What are the major challenges you're seeing in the broader healthcare or manufacturing ecosystem?
Clinard: From a regulatory perspective, the larger the medical device manufacturer, the more competitive the marketplace, the greater the demands on biocompatibility requirements both domestically and internationally.
Qmed: What are you most excited about technologically?
Clinard: Our company's interventional products are minimally invasive and are changing the way doctors approach patient care. It is exciting to be a part of developing advancements and innovations in medical devices that provide the ability to save people's' lives, allow for fewer invasive surgeries and quicker recoveries.
Learn more about the medical device industry at MD&M East in New York City, June 9-11, 2015. |
About the Author(s)
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)
