In 5 Years, Medtech Firms Will Outsource Design, Legal, and Regulatory Services Over Manufacturing, Report Finds
In the largest study of its kind, a new report from MD+DI and ITG Market Research finds that 30 % of medical device companies think outsourcing is essential.
February 26, 2013
The medical device industry is heading into an era of change and uncertainty. Device OEMs are grappling with pressures related to cost, time to market, and increasingly stringent regulation. To remain competitive, OEMs will turn to outsourcing.
Completed on December 30, 2012, the "Medical Device Outsourcing Landscape Study" surveyed 853 respondents from MD+DI’s readership about outsourcing habits, how those habits may change, and the requirements related to contract manufacturing. The full report results are available for purchase (see sidebar).
 Device Industry Threats and Challenges
Device Industry Threats and Challenges

Key Medtech Outsourcing Numbers |
|---|
853Number of medical device professionals respondents.47%Indicated they will focus internal resources on R&D for new product development.28%Said they will increase manufacturing efficiencies for cost effectiveness.31%Said paying the Device Tax was a threat to profitability.* Based on questions asked in the Medical Device Landscape Outsourcing Study, Current and Future Trends: 2013-2018 from MD+DI and ITG Market Research. |
A majority (52%) of device professionals identified regulatory pressures as the most concerning issue they will face in the next five years. Regulatory hurdles are particularly worrisome because they can impede time to market, which 49% of respondents also classified as “concerning” or “very concerning.” Factors such as protecting intellectual property and funding R&D also ranked high among respondents’ anticipated worries.
The device excise tax and healthcare reform weigh heavily on device professionals’ minds. This worry is evident in that 30% of respondents characterized the medical device tax as “very concerning,” while 28% echoed the sentiment for Obamacare. Respondents indicated that paying the excise tax poses the most significant threat to profitability during the next five years.
Current Outsourcing Habits
Cutting costs and expediting time to market remain paramount for OEMs. And the majority of them view contract manufacturing as a means of achieving these two objectives. In fact, 62% of respondents concurred that contract services can save cost and time in certain situations, while 29% find them essential to reducing costs and time to market. An overwhelming majority of respondents further reported that their firms opted to outsource at least some projects.
Of the respondents whose firms rely on outsourcing to at least some extent, 48% acknowledged an uptick in outsourcing over the past five years, and 39% reported that the use of contract services has remained the same during that same period.
The Future of Medtech Outsourcing
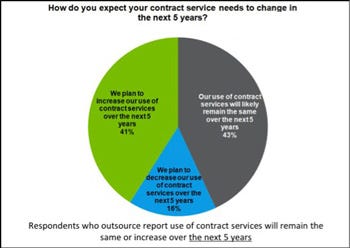
Looking ahead to the next five years, 41% of respondents predict that their companies will increase use of contract services, while 43% speculate that their outsourcing needs will remain unchanged. There is a notable shift in the types of services that will be outsourced in the future, from primarily manufactring services to legal and idea-driven services. Respondents foresee an increased need for contract legal and regulatory services to help cope with the growing regulatory pressures. The also indicated growth for R&D and design capabilities. In anticipation of such needs, some contract manufacturers have already begun to institute formalized design support services, thereby signaling a shifting focus from production services to services that will help medical device manufacturers weather the challenges ahead.
No conversation about contracting services is complete without discussing the issue of outsourcing to foreign versus domestic partners. Yet, nearly three-quarters of respondents have outsourced projects within the United States (73%) during the past five years, indicating continued support for U.S.-based service providers.
Companies do continue to seek out low-cost manufacturing options outside of the United States, however. Among those who already outsource in the countries, Russia (69%) and Latin/South America (61%) topped respondents’ lists as the regions they most expect to expand outsourcing activity in the coming years.
Partnership Expectations
Good vendor relationships are critical to a successful project. Luckily, 65% of medical device manufacturers feel that contract partners meet expectations most of the time. However, digging deeper into these data, the most important aspects of a partnership were also the most challenging to do well (e.g., communication and transparency). The full report identifies those headaches and the top attributes that make partners trustworthy and valuable.
Dive Deeper
Purchase the full report* for a comprehensive look into this insightful study. The 94-page slideshow format provides indepth information and makes it easy to extract information for company presentations Download the order form or contact Becky Roll, Western Regional Sales Manager, UBM Canon MedTech Group to order the Medical Device Outsourcing Landscape: Current & Future Trends, 2013-2018 Report Interested Price: $499
—Heather Thompson is editor-in-chief of MD+DI.
Related Content
The U.S. Medical Device Industry in 2012: Challenges at Home and Abroad
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
