How an EMS Firm and a Lean Startup Are Taking on Chronic Pain
April 18, 2014
|
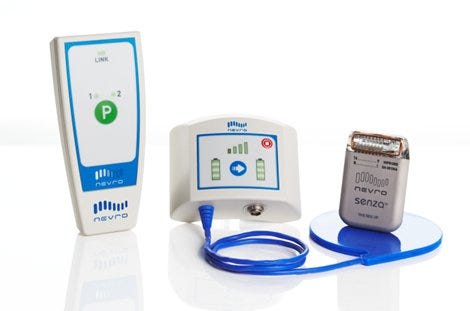
The Senza high-frequency spinal cord stimulation technology could offer relief to many patients with chronic pain. |
In many ways, neurostimulator maker Nevro (Menlo Park, CA) functions like a quintessential Silicon Valley lean startup. The company has embraced the concept of rapid iteration, and was already working on the 10th generation of its pain-relieving neuromodulation technology before taking the product to the market. After receiving CE Mark approval to commercialize the technology in Europe, the company enlisted the help of contract manufacturer Hunter Technology (Milpitas, CA), enabling it to produce and ship more than 1000 units in two years. Longer term, the startup hopes to obtain FDA approval for its technology and ultimately claim a significant piece of the $1.5-billion global spinal stimulation market and provide an alternative to some pharmaceutical and surgical therapies for treating chronic back and leg pain.
As a budget-minded startup that is working on obtaining FDA approval for its implantable Senza device, the partnership with Hunter helped Nevro to streamline the process of integrating design changes while meeting ISO 13485 requirements.
MPMN recently had the opportunity to tour Hunter's 62,500-sq-ft electronics manufacturing services (EMS) facilities in Milpitas. During the visit, Hunter and Nevro executives explained how the two firms collaborated in the development of the Senza platform, which generates up to 10,000 electrical pulses to the spinal cord every second--a rate many times faster than traditional low-frequency spinal-cord-stimulation (SCS) devices. Nevro's technology is said to be both more effective than traditional devices, while also eliminating paresthesia--an annoying buzzing sensation caused by conventional SCS devices.
Refresh your medical device industry knowledge at MD&M East, June 9-12, 2014 in New York City. |
Partnering with Hunter helped keep the startup nimble, explained Nevro's founder and senior vice president Andre Walker. Hunter oversees roughly 100 unique manufacturing projects per week. It takes the company about 20 minutes to shut down a production line and retool it to produce a different product. A team of 15 full-time process engineers helps make that happen by constantly churning out build instructions.
Similarly, Nevro had been developing four prototypes at once before settling on the tenth-generation of the technology it intended to commercialize. "We had a new version every two to three months," Walker said.
Working with Hunter is enabling Nevro to focus on its pivotal trial needed to ultimately commercialize its technology in the United States. When the Nevro was founded, Walker assumed it would take about three years to commercialize its device in the United States. But the regulatory rules changed midstream, he says. Now, the company is about seven years in, and is now in the middle of a pivotal U.S. study with a price tag thus far of upwards of $90 million.
It helps that the company was fortunate enough to begin receiving millions in funding before the financial meltdown of 2008. And the company has obtained several rounds of funding since, including from the likes of Johnson & Johnson, Covidien, Mayo Clinic Ventures, and several other VC firms.
Still, the company is doing everything it can to preserve its cash for essential functions. "A startup should never be in the position of needing money," explained Nevro's founder and senior vice president Andre Walker. Instead, the firm should have a singular focus on developing the best-possible product--one that investors will prize. Accomplishing that goal is no easy feat, of course, and the shifting regulatory climate has been difficult and expensive for medical device firms to navigate.
The partnership with Hunter has been helpful in that regard because the contract manufacturer is well versed in medical device production. The biggest market the firm serves is the medical device sector. Hunter is certified to ISO 13485 and expects to certify its quality management system to 21 CFR 820 requirements.
The EMS provider, which has a strong engineering front-end, is also helping Nevro integrate design changes, while churning out new devices as quickly as possible to meet market demand.
Brian Buntz is the editor-in-chief of Qmed and MPMN. Follow him on Twitter at @brian_buntz and Google+.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
