BIOMEDevice San Jose Innovation Prize: Meet the Finalists
November 16, 2016
Discover some of the most innovative exhibitors at the upcoming BIOMEDevice San Jose show.
Chris Newmarker
From more reliable nitinol to enabling MRI-compatible catheters to simplified catheter packaging, there are a wide variety of innovations among the five BIOMEDevice San Jose innovation prize finalists chosen by UBM editors and readers.
Check out the five finalists yourself on Wednesday, December 7, at 1 p.m., when MD+DI managing editor Marie Thibault leads an Innovation Tour out of Center Stage at BIOMEDevice San Jose. You'll be able to vote for the Innovation Prize winner at the end of the tour.
 Here are the five finalists:
Here are the five finalists:
1. Enabling Nitinol Innovation
Lumenous Device Technologies (Sunnyvale, CA)
Booth 1011
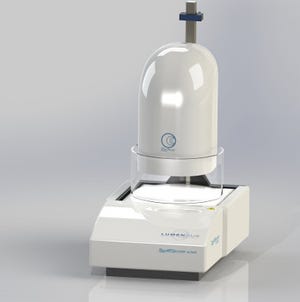
Nitinol's superelastic behavior depends on its transformation temperature (Af) to the superelastic phase. The Af must be carefully controlled for safe and effective medical devices, but Af testing has been difficult to perform in the past. Enter Lumenous with its TruePhaseTM, developed through a partnership with Stanford University. TruePhase uses advanced vision technology to determine the transition temperature of nitinol. And it does so without having to physically touch the material. Says Lumenous: "Better data can even enable longer fatigue life on implantable devices."
Lumenous TruePhase Af Measurement Solution from Todd Dickson on Vimeo.
 2. Making Catheters MRI-Compatible
2. Making Catheters MRI-Compatible

Zeus (Orangeburg, SC)
Booth 620
Launched in September 2016, Zeus LCP monofilament is meant to enable MRI-compatible catheters--something that medical professionals are increasingly demanding as minimally invasive medical procedures evolve. The high performance specialized fiber is meant to replace the SS wire that has been used in reinforcement braiding in catheters intended for minimally invasive procedures. LCP boasts torquability and stiffness, but it also allows deflectability at the distal end of the device
Here's how Zeus describes the creation of the LCP monofilament: "Zeus LCP monofilament is braided over the catheter base liner during construction to give mechanic strength and support for the finished catheter. The braiding is then covered with a jacketing material such as Pebax or nylon which is reflowed under a layer of heat shrink. Reflow during the heat shrink step allows the jacketing to melt and flow between the individual catheter braiding strands and bond to the underlying base liner. The result is a firm finished catheter with the jacket and base liner fused with the braiding between them providing structural reinforcement."
 3. Creating Tiny Bioabsorbable Components
3. Creating Tiny Bioabsorbable Components

MTD Micro Molding (Charlton, MA)
Booth 1014
MTD Micro Molding touts that it is able to reliably create tiny, delicate, and intricate bioabsorbable components required for specialized surgeries, orthopedic devices, and advanced delivery devices. MTD is able to achieve this in a number of ways:
MTD's MicroRunner tool has a ratcheting runner system. It varies in diameter and helps determine the minimum runner size required to fill the volume of a part, adequately molding a product without sacrificing material.
MTD is able to fully document and customize its validation processes for each client and project.
The company also boasts in-house inherent viscosity testing. Says MTD: "A micromolding company should have IV testing capability on-site, so the impact of process variables on IV can be determined and adjusted immediately."
MTD has the in-house capability for real-time, continuous testing.
MTD stores bioabsorbable and sensitive materials and molded inventory in temperature controlled environments.
Specialized micromolding equipment effectively controls critical bioabsorbable processing factors. These include residence time, shear, and degradation rate of material.
 4. A Medical Device Coating That Bonds at an Atomic Level
4. A Medical Device Coating That Bonds at an Atomic Level

ProPlate (Anoka, MN)
Booth 100
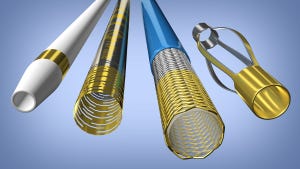
ProPlate's Vizi-Band is a radiopaque marker coating innovation. It atomically bonds a metal directly to a catheter, guide wire, stent, or similar component, and can be applied selectively. ProPlate boasts that ViziBand, which launched in late 2014, does away with risks associated with more traditional methods such as metal-filled polymers or machined bands. Metal-filled plastic may suffer from long-term storage degradation, according to ProPlate. Go the machined bands route, and there may be dislodgement of bands crimped onto the device.
Says ProPlate: "There is a process reduction with the use of Vizi-Band coating technology that can create cost savings in both labor and materials. Whereas traditional machined marker bands are laboriously crimped or swaged onto catheters, Vizi-Band markers are coated directly to the medical device and can be accomplished in large volumes simultaneously for maximum efficiency. Where radiopacity requirements are lower, Vizi-Band markers can be applied thinner than traditional marker bands are machined, which will impart a performance advantage of a lower profile while reducing material costs. Additionally the risk of dislodgement is eliminated because Vizi-Band is bonded at an atomic level."
 5. Simple Packaging for Catheters and Guidewires
5. Simple Packaging for Catheters and Guidewires

CleanCut Technologies (Anaheim, CA)
Booth 429
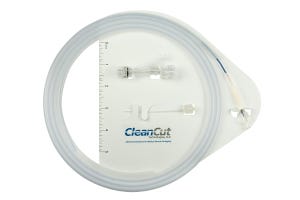
CleanCut Technologies boasts that its DISK is a convenient "all-in-one" packaging system for catheter and/or guidewire devices along with procedural accessories in a convenient "all-in-one" system. Says CleanCut: "Developed in 2014, the DISK is created by bonding, CleanCut Technologies one-piece 'Clipless Guidewire and Catheter Dispenser' to an HDPE backer card, without glue or adhesives. The DISK does not use any clips but instead bonds HDPE tubing in various ID/OD's and lengths up to 217 in. Without the need for clips, as with traditional catheter packaging, material is reduced by 20-30%, resulting in significant cost savings. Medical professionals have found the DISK to be exceptionally convenient during surgery by reducing prep time and the need to open and assemble individually packaged devices."
This DISK system provides a surgical team with a single packaging delivery system, versus multiple sterile pouches containing individual components needed to complete a procedure.
Chris Newmarker is senior editor of Qmed. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like


