5 Ways to Ensure Your Medical Device Customers Are Taken Care of after Launch
May 28, 2015
It is important to have a solid product support strategy in place before your device hits the market. Here's how you do that.
Qmed Staff
|
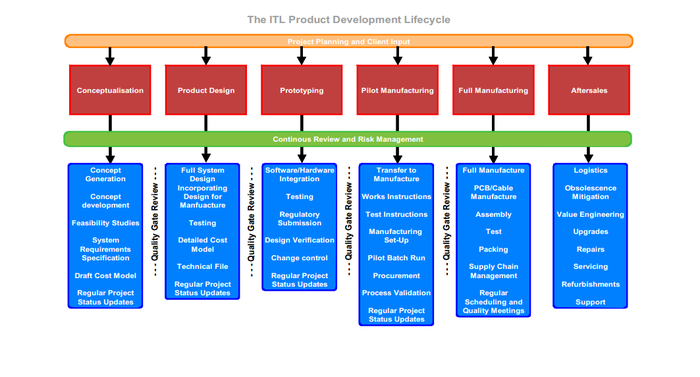
(Image courtesy of Integrated Technologies Ltd.) |
It is one thing to develop a medical device product. It is quite another thing to move into the phase where the product is actually being used, and customer support is required.
It all requires careful strategic planning, according to Integrated Technologies Ltd. (Ashford, United Kingdom), the European Headquarters of the ITL Group.
Unfortunately, ITL has noticed that it is fairly common for a project to be launched without a comprehensive support and after-sales service in place. That leads to a huge amount of risk, both from a user/patient safety and market acceptance perspective, says ITL, which provides a global Medical Device product development, product design and contract manufacturing service.
ITL Virginia recently posted an article on its website that includes five steps that can be taken at appropriate times throughout the design and development of a medical device product to ensure that customers are fully supported post-launch. Here is a summary of some key points, with added comments from Thomas Jull, vice president of operations at ITL Virginia:
1. Ensure Spare Parts Are Available for Later Stages
When ordering parts for the first batch of units to be manufactured (or during pilot production), it is important to order extra spares to allow for units that may get damaged or malfunction, according to ITL. Some spare parts can have lead times as long as 12 to 16 weeks. Custom fabricated parts sometimes take even longer to make. So it may save a company some money in the longrun to include some spares in the initial manufacturing order, at lest when it comes to parts with long lead times.
An extra smart step is to have an assembled and functioning "replacement" unit in stock, adds Jull. "Not only does such a unit allow for a quick temporary or permanent swap-out, but it can also be used back at base to physicially replicate faults or visualize potential solutions," Jull says.
2. Understand the Location and Distribution of Customers
Frankly, your biggest market or favored product launch site might be a long way from your company's headquarters.
Says ITL in its article: "Supporting users remotely for non-hands-on tasks may be OK for small volumes but this will come down to the type of product and customer. Jumping on a plane to talk to customers face-to-face or to physically inspect a device for faults can sometimes be the only way to sort out problems. If, however this is on the other side of the world from headquarters, then expenses and time spent can soon build up. ... Ask the question, 'Will we be able to provide effective onsite and remote customer support ourselves?'"
It is important to remember that culture and perspective toward use of a device could vary by region, so it is important to gain an understanding of this to form an effective aftersales strategy.
3. Confirm Your Logistics, Import, and Export Strategies
Here's the next major question: How are you going to get product to your customers?
It starts with device desigh, including additional shipping components removed during installation to cope with transportation, type of shipping (air, road, sea, etc.), and good packaging design, according to ITL. It is a good excercise to obtain quotes from couriers/brokers before launch, and eecide whether to pass the charges onto the customer or absorb yourself."
Adds Jull: "Make sure you know what and how you are shipping before you send it off. If you get it wrong it might be the last time you see it."
Import/export strategies including understanding import taxes and proper customs documention is also key, as is an efficient warehousing strategy.
4. Set Up Good Communications
An effective after-sales strategy cannot function without good communications. It includes having local technical support and product services in the same time zone and language as your customers, according to ITL. Remember that some clients may require 24-hour access to such features, and may demand fast turnaround times. is essential to keeping the customer happy and for dealing with aftersales requests such as technical support and product servicing.
Information can be effectively shared among relevant stakeholders through emailing of reports, including data downloaded from devices and customer observations.
Don't ignore all the recent advances in communications technology, says Jull. "With the ability to be connected to the Internet via a cell phones now a days and with video conferencing apps like Skype mobile, a technician can be sent to a customer site to diagnose and fix faults on a unit and have the backing of the whole team watching the stream and giving direction from the office. Utilization of such technology and others like remote access software for PC based products should be considered when planning a strategy."
5. Implement Device Tracking Systems/Processes
There needs to be a tracking system used for collecting data on products, tracing units, recording customer feedback/complaints, and decision making for actions. "As with all of the above, this is not something that should be planned on an ad hoc basis during launch (or worse, when the first units come back to you for repair)," ITL says in its article. All competent OEMs should have Quality Management Systems (QMS) in place.
"It is also important to have unit consistency and traceability between you and your customers," Jull says. "Customers may need to be educated on how to report faults and what data to use. They may also need an appropriate format for such record keeping and it may be a good idea for you to supply them with one. It is likely that if a customer has many of your products, you will both be using similar data and so having a similar format for device tracking makes sense, especially if you are sharing information."
Refresh your medical device industry knowledge at MD&M East in New York City, June 9-11, 2015. |
Chris Newmarker is senior editor of Qmed and MPMN. Follow him on Twitter at @newmarker.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
