Updated Blood Collection Device Draws 510(k) from FDA
February 28, 2017
FDA cleared Velano Vascular's PIVO needle-free blood collection device, which attaches to a peripheral IV system.
Nancy Crotti
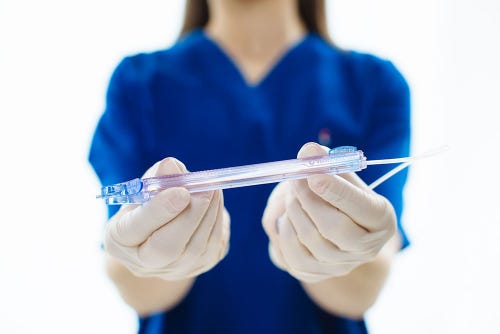
The PIVO advances an inner tube via a slider mechanism through the peripheral IV catheter system.
Velano Vascular has won 510(k) clearance for a next-generation version its blood-draw device aimed at reducing painful needle sticks.
The PIVO, formerly called TIVA, is a single-use, needle-free blood collection device that attaches to a peripheral IV system.The device, which comes in 20- and 22-gauge sizes, advances an inner tube via a slider mechanism through the peripheral IV catheter system. PIVO possesses a proximal flexible tube with female luer that attaches to a blood transfer device or syringe, into which a blood sample is collected.
FDA, which helped with development of the original device through a grant to the New England Pediatric Device Consortium, approved the first version in early 2015. The San Francisco--based company spent 18 months working on updates that would make it easier to use and scalable for global distribution, according to co-founder and CEO Eric Stone. The PIVO is simpler and requires fewer steps for nurses, phlebotomists, and medical office staff.
"We removed a significant number of components," Stone told Qmed. "We removed an adhesive with multiple bonds, and we redesigned for full automation."
The device is in use in six or seven hospitals or health systems in the United States, he noted. Both versions have received the CE mark, and Stone said the company would be ready to discuss global distribution later this year.
The 20- and 22-gauge PIVO devices may be used with adult patients, while the 22 works with some children and with adults. Velano is working on developing a 24-gauge size for smaller children.
"The results in pediatrics are certainly promising," Stone said. "I very much expect we will develop this procedure as standard of care for pediatrics."
Stone knows how much pain and anxiety needle sticks can cause patients, especially pediatric patients. As a child with Crohn's disease, he underwent multiple hospitalizations and many needle sticks.
The company designed the first version of PIVO to show that it could come up with an effective technology to produce strong blood draws from a peripheral IV, according to Stone. Now that it has done so, Velano wants to make that technology more widely available.
"First and foremost, to develop a new standard of care requires observing, listening, asking, learning, and iterating," Stone said. "In order to establish the standard of care for such a broad-based and pervasive procedure, the technology has to be scalable from a usability perspective and from a manufacturing perspective, so we can meet demand."
Nancy Crotti is a freelance contributor for Qmed.
[Photo credit: Velano Vascular]
About the Author(s)
You May Also Like


