Unraveling Misconnections in Medical Tubing
TUBING - ONLINE EXPANDED VERSION
March 1, 2009
Click to enlarge |
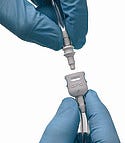
Some tubing connectors are designed to eliminate potential misconnections that can occur with luer fittings. |
A 24-year-old woman was nearly nine months pregnant when she was hospitalized for vomiting and dehydration. A nurse was supposed to connect a bag containing a nutritional solution to the woman's feeding tube to provide her with nutrients. Instead, the nurse mistakenly connected the feeding tube to the patient's IV line and injected the nutritional solution directly into her vascular system. Shortly after the mistake, the mother and her baby died.
This medical mix-up underscores the potentially devastating consequences of a persistent and often underreported problem in hospitals and healthcare facilities across the country: tubing misconnections. The culprit behind many of these tubing tragedies is a device called a luer connector. Used in many kinds of medical equipment, the luer was originally created to standardize the way hypodermic needles and syringes join together. The luer allowed different needle and syringe manufacturers to make widely compatible products and thus broadened the market for both devices.
Although there is not yet a universal way to prevent tubing misconnections, manufacturers should explore different connectivity options in their medical device designs. In addition, they should determine whether designing devices with incompatible connections may help solve the misconnection problem.
A Universal Fit Creates a Persistent Problem
A luer connector is a small, plastic fitting with standard male and female geometries that lock into place. It offers no tactile feedback to indicate that an error was made because the connectors fit together easily.
Due to its simplicity and familiarity, the luer connector has since become the universal method of joining not only needles and syringes, but lengths of small-bore medical tubing that are a mainstay of modern medical equipment. However, the uniformity offered by luers can cause problems because they allow users to mistakenly connect a broad range of devices that have completely different functions.
Although many errors are caught in time to avoid harm, the nonprofit Joint Commission, a hospital accreditation agency, issued an alert to healthcare organizations in 2006, warning that misconnections are a “persistent and potentially deadly occurrence.” The alert cited nine cases of misconnections, eight of which led to death, and another to serious disability.
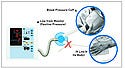
In one case, the tubing from a portable blood pressure monitoring device was mistakenly connected to the patient's IV line, and the patient died from an air embolism. In another case, an air supply hose from a pneumatic compression device was inadvertently hooked up to a needleless IV tubing port. Fortunately, the mistake was caught in time.
More than 1200 times in the past 10 years, U.S. hospital workers have inadvertently connected tubes meant to link one device or system—an IV, a feeding tube, a catheter—into another device, frequently causing harm and sometimes death. But these figures may represent just a fraction of the total incidents. That's because they are based on voluntary, anonymous reports from only 15% of the country's 5800 hospitals—the 875 facilities that participate in an error-sharing program created by U.S. Pharmacopeia, a pharmacy standards agency.
The Joint Commission is one of the many healthcare organizations that have called for a solution to the problem. But developing a workable, worldwide industry standard is a complicated process that requires painstaking review and examination of various concerns. Meanwhile, manufacturers should try to develop alternatives with the aim of saving lives.
Even as concerns mount and new cases emerge each year, the nation's healthcare system may still be years away from developing what would appear to be a seemingly simple solution.
One barrier, which exists with any type of patient-safety solution, is that it's often unclear whether regulators, equipment manufacturers, or hospitals should be responsible for effecting change. Jay Crowley, a senior adviser for patient safety at CDRH, also said that there is no strong incentive among OEMs for any specific type of solution.
During the past three decades, a number of OEMs have attempted to address tubing misconnections using novel adapters, nonstandard connectors, and other unique product designs. Manufacturers have introduced connectors of varying sizes with different mating functionality that prevents them from being connected to luer fittings. Not only have these products not been easy to use, but many of them simply didn't work—they leaked.Without a dedicated standard to support or encourage the use of nonluer connections, many of these products were not successful in the marketplace. Currently, no published standards specifically restrict the use of luer connectors on certain medical devices, with the exception of blood pressure cuffs.
The Path toward Progress
Click to enlarge |
(click to enlarge) Tubing from a blood pressure monitoring device can be mistakenly connected to the patient's IV line, causing serious injury or death. |
In 1990, the European Committee for Standardization (CEN) established a group of technical and clinical experts to explore solutions to help prevent misconnections. Among the early developments was a standard outlawing luers in blood pressure cuffs.
In 2001, CEN initiated an effort to examine six different applications that used luers and sought to create unique connectors for each. The design of each male-female pairing would ensure that it could not be attached to any others. After examining the issues more closely (and perhaps seeing the enormous complexity and multitude of potential unintended consequences), the group took no definitive action and produced no new industry standards.
AAMI has also explored ways to prevent misconnections. In 1996, the group convened an expert team to address the safety requirements for enteral feeding set connectors and adapters for the delivery of fluid directly into the gastrointestinal tract. The resulting voluntary standard, approved in 1996 and reaffirmed in 2005, recommended that adapters and connectors used in an enteral system be incompatible with female luer-lock rigid connectors. However, no alternative design standards were ever developed or approved on the basis of that document. And because the standard is voluntary, it is not universally followed by all device manufacturers.
In 2007, AAMI, along with FDA, began to transition the standards-development work for medical tubing to the International Organization for Standardization (ISO). Although ISO has no enforcement authority, its standards often become law, making it more powerful than most nongovernmental organizations.
ISO is considering a proposed series of international standards that would cover the general requirements for small-bore connectors for liquids and gases in healthcare applications. In addition, various ISO committees have begun examining the use of luers in other core medical device applications, including limb cuff inflation systems as well as vascular, enteral, respiratory, neuraxial and urethral/urinary systems. However, there is no timeline set for the ISO standard to be published.
A consensus among those involved in the standards development process is that vascular IV lines will likely (and should continue to be) the exclusive domain of luers. The goal then would be to ensure that incompatible devices will be unable to connect to an IV line. That would mean that no other equipment in a hospital setting around a patient would be able to use a luer as a connector. Questions that naturally follow from this include: How will all of the other applications be addressed? Who would design the new connectors? How would authorities test and approve the effectiveness? Who has the final decision? These complications make the solutions less clear.
Make It Different
Patient safety advocates say the tubing problem has a simple solution: Create different connectors for different kinds of tubing, making it nearly impossible to link incompatible devices and systems. Indeed, other industries have found success in making things incompatible. For example, petroleum producers prevent fuel from flowing into the wrong kind of gasoline tank by using different pump nozzles for diesel and regular gas.
The Joint Commission has urged product manufacturers to implement “designed incompatibility” to prevent dangerous tubing misconnections. More specifically, it recommends a change to a forcing function design as a way to best avoid errors. A forcing function design would make incorrect connections impossible because it would physically prevent the user from taking a harmful action. For example, a syringe filled with a drug intended for an intravenous infusion would not fit with any other medical connector, such as an air supply hose or feeding tube.
But even device incompatibility is not the end-all solution that it may appear to be. Federal regulators welcome the idea of having a variety of connectors, but they say the complex dynamics among regulators, manufacturers, and hospitals create additional challenges. For example, within the United States, there is no easy way to force manufacturers to create new connectors. FDA could require the use of particular connectors, but that is more difficult than encouraging manufacturers to take the initiative. Therefore, it's important for hospitals and other healthcare organizations to collaborate with equipment suppliers and manufacturers to explore new designs and innovations that can help prevent tubing misconnections.
In addition, any singular solution potentially affects a wide variety of equipment. For example, if connectors are created for different kinds of tubes, syringes, catheters, and other devices that connect to those tubes, the tubes would also need to be changed. Such action would require the cooperation of many different manufacturers.
Without a defined standard for all of the points of connection for a particular device, manufacturers will be severely challenged to create products that interface with parts that they do not manufacture. For example, companies that make feeding tubes are not necessarily the same companies that manufacture enteral formula bags or feeding pump sets.
Striking a Balance
There is also concern about the need to strike an optimal balance between safety and flexibility. Doctors and nurses frequently want to be able to connect a lot of equipment—especially in emergencies, when they may not have time to search for specific types of tubing. According to Crowley, it's crucial that any standards that are developed aren't so restrictive that they become a liability, hindering the speed and efficiency required in these critical environments.
As the standards development process continues to move forward, many hospitals have already begun to take action by improving their labeling of different kinds of tubing and using IV tubes that cannot be attached to feeding-tube syringes. But some of these approaches have the potential for unintended consequences. For example, color-coding tubes and catheters can lead users to rely on the color rather than visually inspecting which tubes are connected to which body inlets. In addition, color-coding schemes are not conducive to conditions in which there is minimal light, and such schemes often vary across institutions in the same community.
OEM Challenges
At the same time, medical equipment OEMs are reevaluating their connector choices as they look for suitable luer alternatives. In the past, when design engineers needed a connector, they would simply take a standard luer off the shelf with little worry about potential patient safety or product liability issues. Luers were well understood, widely used, and universally accepted.
Now as designers are forced to move away from the familiar luer, they not only have to think about the technical aspects of the connector in terms of flow rates, pressures, and ease of use, but also about how the device could potentially interface with an unknown number of yet undefined devices that could be in the marketplace.
In addition, as plastics manufacturers develop and combine various resins to make new materials, designers need to consider compatibility of the connector material to prevent incompatibility with different fluids and gases, or contamination. Connectors are typically made of metals such as stainless steel, brass, aluminum, or zinc, or plastics such as PTFE, PFA, polycarbonate, or polysulfone. Common luer materials are polycarbonate, polypropylene, and nylon, among others.
These variables, along with the uncertainty of how the standards will ultimately take shape, bring new challenges to the medical device design process. Although some OEMs excel at designing and manufacturing medical equipment, they may not be experts at making safe, reliable, and cost-effective connectors. For medical device designs that may eventually require connectors, OEMs should consult an experienced connector manufacturer. These manufacturers can play an important role in ensuring that the new connector will meet the OEM's specific performance, reliability, and safety requirements.
OEMs should look for a supplier with a solid history of connector design and application expertise. In scrutinizing potential suppliers, OEMs should consider several key questions:
Has the supplier designed connectors for medical applications before?
Does the company have products in the marketplace?
Is the supplier familiar with the intricacies of connector design and manufacturing, or is it just a plastics or fittings molder?
Does the company have knowledge and experience with tubing misconnection issues?
Some connector manufacturers have already begun exploring effective luer alternatives. For example, there are a variety of small-bore connectors designed for a wide range of liquid-media and gas applications, such as blood and bodily fluid–handling and air-driven devices. Such connectors not only eliminate the potential for dangerous misconnections with luer fittings, but they also feature a more-secure latch design with a distinct, audible click. The connectors allow tubing rotation to help prevent accidental disconnections or kinked tubing.
Manufacturers, and especially designers, should explore fail-safe connector technologies to integrate into their medical equipment. Many of these technologies are still in the development stage. But ultimately, OEMs should lead the way in the battle against medical tubing misconnections.
Jim Brown is business unit manager for medical markets at Colder Products Co. (St. Paul, MN). Reach him at [email protected].
Copyright ©2009 Medical Device & Diagnostic Industry
About the Author(s)
You May Also Like