Developing balloon catheters with lower profiles will be key to advancing transcatheter aortic valve replacement systems.
October 21, 2014

By Kenny Mazzarese
Positive clinical study outcomes for transcatheter aortic valve replacement (TAVR) procedures have increased the size of the market for TAVR devices while attracting fierce competition. Today, at least six medical device companies are in the process of seeking approval for TAVR or transcatheter aortic valve implant (TAVI) devices.
As these manufacturers race to develop next-generation products, they are pushing their suppliers to deliver better balloon catheters, a key component in TAVR systems. Improvements in the size, compliance, burst pressure, profile, and cone angle of balloon catheters will be pivotal in continuing to improve TAVR technology. But among these characteristics, profile is perhaps the most important. A TAVR balloon catheter with all of the same performance characteristics but smaller sheath compatibility than a competing product would be considered the product of choice in the market.
How Balloon Catheters are Used in TAVR
|
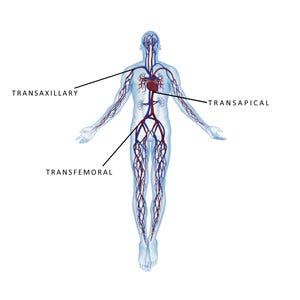
Figure 1. For TAVR procedures, the access site can be transfemoral, transapical, or transaxillary. |
Balloon catheter delivery systems are the single most designated therapy delivery device in the majority of intra-arterial procedures. In TAVR procedures, they are used to enlarge narrow arterial pathways to enable delivery of the valve. They also play an essential role in native valve preparation, artificial valve size selection, and sometimes post-valve implant fit.
Endovascular procedures can be performed through various anatomical access sites, presenting entirely different vessel diameter requirements and anatomical hemodynamics that must be considered in selecting optimal device delivery systems. For TAVR procedures, the access site can be transfemoral, transapical, or transaxillary (Figure 1) . Access site selection is based on the age of the patient or contraindications to the relevant disease state and cofactors determining valve characteristics, diameter, tortuosity, viscosity of blood, and blood pressure of the artery selected.
The first-generation TAVR device used a 22–25-Fr sheath introducer delivered via groin puncture for transfemoral access. Contraindicated patients and those with chaotic vessels and fatty deposits within the anatomy posed challenges to interventional cardiologists. For such patients, the transapical technique utilizing balloon-assisted valvuloplasty (BAV) became the method of choice. This method has the potential to reduce the incidence of complications by facilitating single operator use.
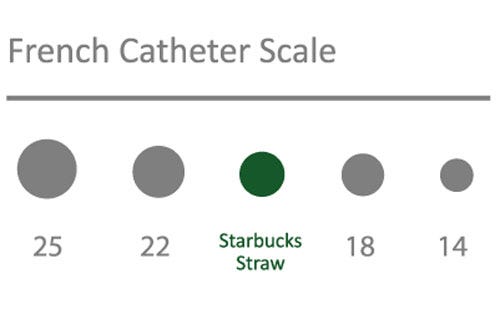
Successive TAVR device iterations are demanding balloon catheters with a lower profile. Specifications now call for compatibility with 18-Fr sheath introducers, and next-generation 14-Fr sheath introducers that are balloon expandable to 18 Fr propose requirements for even lower balloon profiles (Figure 2).
Achieving a Low-Profile Balloon Catheter
From a manufacturing standpoint, this continuing evolution presents new challenges for device process engineers. The single most important consideration in designing a balloon catheter for endovascular access and delivery is the profile of the complete system. Key areas of manufacturing that determine overall balloon catheter profile include balloon wall thickness, weld, and fluting and wrapping. Reducing the wall thickness of the balloon while maintaining the desired growth compliance and burst pressure requires the use of specialized materials, balloon extrusion processes, and balloon forming parameters.
|
Figure 2. Balloon catheter delivery systems for TAVR/TAVI have continually reduced in profile to as low as 14 FR. |
The area of the balloon that usually has the highest profile is the proximal tail outer diameter weld. A thermal compression weld of the balloon tail to the shaft helps reduce the profile of the weld by axially displacing the molten material. Conventional weld techniques rely only on the radial compression of the heat shrink and can only displace so much material, whereas the latest in catheter weld equipment has improved to include welding jaws that apply a much higher force over a longer area.
The fluting operation, essentially a precision pleated fold of the balloon around the catheter shaft, is tailored to each individual balloon. The number of flutes, or pleats, used is critical for proper material displacement to minimize unnecessary balloon wall overlap. The fluting machine, designed to thermally iron pleats into the balloon, works to include the entire length through to the cone of the balloon and extending down the balloon tail to determine the outside diameter specification. The thickest portion of the balloon is the cone at the tail transition. If this portion of the cone is not fluted and wrapped properly, it will be difficult for the balloon to achieve the desired profile.
Involving process engineers in the balloon catheter design phase helps to mitigate potential problems that can impact critical time-to-market deadlines. While balloon design specifications can translate from a device engineer’s drawing to a physical prototype, repeatability in manufacturing must be established early in the design review. Considering tight dimensional tolerances, which are becoming increasingly confined for new balloon catheter applications, during the development of the manufacturing process will determine if such constraints are necessary and where there is room to enhance the design for ease of exacting throughout production cycles. With checkpoints for product and validation testing implemented during the planning process, management teams can focus on clinical trials, marketing, and clinical training programs rather than production.
Conclusion
While technological advances in arterial device delivery systems have diminished the risk of procedural access complications, access complications still exist in patients with severe calcified stenosis and other anatomical reasons for non-navigability of current catheter delivery systems. Balloon catheters can help to avoid major hemodynamic complications in endovascular surgical procedures and will continue to develop in concert with the complexities of new vascular device technologies such as TAVR. The challenge for next-generation devices is to maintain the robustness of the current delivery system while continuing to reduce the profile of the device.
Kenny Mazzarese is director of advanced technologies at Interface Catheter Solutions. Reach him at [email protected].
You May Also Like