Light Curing Adhesives with Confidence
In high volume, automated manufacturing, medical device manufacturers are looking to light cure adhesives.
|
To date, the greatest challenge related to manufacturing with light cure adhesive technology has been easily verifying the adhesive’s degree of cure. Thirty years ago when the adhesive industry introduced acrylic-based adhesives that solidified on exposure to UV light, the technology offered medical device manufacturers distinct advantages over traditional adhesive technologies such as cyanoacrylates and epoxies. Light cure adhesives delivered very rapid cure, were compatible with a variety of substrates, filled large gaps, and were easy to automate. As a result, medical device manufacturers could accelerate their production processes and produce aesthetically pleasing, clear bondlines.
Even the most conscientious device manufacturer has experienced incomplete cure during production. Light cure adhesives can fail to cure fully when any part of the bond line is inaccessible to light generated by the light source. Failure can also happen when an inappropriate or degraded light source generates insufficient irradiance to complete cure or rapid processing speeds result in insufficient light exposure times.
To address concerns that the appropriate light energy is consistently applied to bonded joints, medical device manufacturers have long desired a quantifiable in-line process that would verify completeness of cure. Even when the adhesive appears solidified and the assembly appears strong, without extensive offline testing that involves either physical destruction of the assembly or time-consuming analytical quality testing, manufacturers have had no way to easily and conclusively confirm the degree of cure on the assembly line.
In-line optical assessment technology allows device manufacturers to quantifiably confirm the degree of cure of light cure acrylic adhesives used in their assembly processes. For example, Loctite AssureCure technology uses light to examine the adhesive bond line and instantaneously provides degree of cure data that correlates directly to trusted off-line cure measurement techniques.
The Light Cure Process
Light cure acrylic adhesives must be exposed to light of the appropriate wavelength and intensity to activate the curing process. The wavelengths of light are found in the electromagnetic spectrum, which categorizes radiant energy by wavelength. Light cure acrylic adhesives are typically cured using ultraviolet or visible wavelengths of light in the range of 200–500 nm. Table 1 is a summary of the major benefits and considerations of traditional light cure adhesives.
|
Table I. Click the table to enlarge it and see the benefits to light cure adhesives. |
Image 1illustrates the sequence of a typical light cure reaction. The uncured adhesive is shown in part A, where the white spheres represent monomer and the double red spheres represent photoinitiators. When the liquid adhesive is exposed to the appropriate light energy, the photoinitiators fracture into free radicals, represented by the single red spheres, as shown in part B. The free radicals then initiate the curing or polymerization process shown in part C, leading to the extremely rapid development of the crosslinked polymer chains that make up the cured adhesive shown in part D.
To confirm the light cure process has properly occurred, manufacturers employ the following traditional cure detection methods:
Fluorescent adhesives confirm only that the adhesive is present in the required areas of the bondline but give no indication that the curing process has occurred.
Color changing adhesives show that the adhesive has been exposed to light, but do not confirm conclusively that the curing process has initiated and progressed to completion by linking color changes to free radical generation or cure reaction.
A physical or destructive testing process confirms that cure has occurred but only for the tested assembly. Since the procedure cannot be integrated in-line, detection is not immediate and a time lag may occur between assembly of the defective part and detection of the problem. Since the manufacturer cannot immediately stop the production of defective assemblies, the value of both the tested parts and the defective parts produced during the delay are lost as scrap.
Analytical testing such as Fourier Transform Infrared Spectroscopy (FTIR) or Differential Scanning Calorimetry can confirm that the part has achieved an acceptable level of cure but cannot be integrated in-line for immediate quality verification. Analytical testing processes result in parts lost to testing and will not prevent a buildup of defective parts if incomplete curing continues.
In-Line Process System Basics
An in-line process such as AssureCure technology provides medical device designers and manufacturers with a nondestructive process to confirm that a light cure acrylic adhesive has cured to the required level for optimum device performance. The system is installed directly on the manufacturing line immediately after the curing equipment. It consists of a specialty light-cure adhesive formulation, a fiber optic light source, optical detection equipment, and a software package. The system optically measures changes during polymerization and applies an algorithm to this measurement to determine the degree of cure. The measurement is obtained in as little as 20 milliseconds.
The fiber optic light source momentarily illuminates the adhesive bondline, and the detection equipment analyzes the response signal. By applying a specially developed algorithm to the response signal, the system provides an accurate analysis of the adhesive’s degree of cure. The data is collected, analyzed, and can be displayed on an existing PC or PLC using the system’s software. Results can be displayed numerically corresponding to the degree of cure or as a pass-fail measurement.
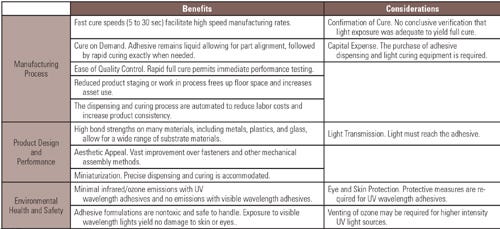
The process of integrating an in-line light cure adhesive detection system on a production line starts by producing correlation curves for the manufacturer’s specific application. The correlation curve shown in Figure 1 compares the adhesive’s degree of cure based on FTIR measurements to the output from the system. Figure 1 shows the correlation between the AssureCure system response on the right scale, and on the left scale, the percent conversion via FTIR analysis of the adhesive over varying light exposure times from 0 to 3 seconds using a 405-nm LED source. As the measurement from the AssureCure system increases, the percent cure or percent conversion measured with FTIR also increases.
|
Percent of cure (conversion %) from FTIR analysis versus AssureCure detector response over varying light exposure time intervals |
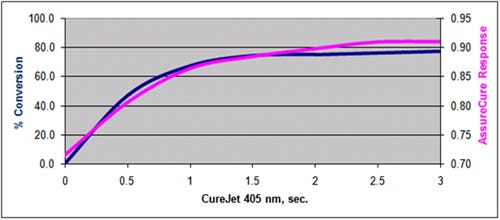
The AssureCure system data also directly correlates to the adhesive’s strength build over time. Figure 2 shows the correlation curve of needle pull strengths versus the AssureCure output over various time intervals of light exposure. As the pull strength of the polycarbonate hub to the stainless steel cannula increases, the data from the AssureCure system also increases, showing excellent correlation. The system provides confirmation that the adhesive has cured and reached the desired strength for the target use.
|
Figure 2 shows the correlation between needle pull strength and AssureCure detector response over varying light exposure time intervals. |
Biocompatibility is essential to ensure that medical devices are safe for their intended end use. Cytotoxicity testing is based on a scale of 0–4 depending on the biological reactivity, or cellular degeneration and malformation, of the test specimen ( the cured adhesive). A result of grade 0 indicates that there was no reactivity, and a grade 4 indicates that there was a severe amount of reactivity.
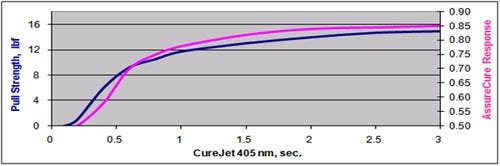
Figure 3 shows a correlation curve that compares the AssureCure output to the biocompatibility of a bonded assembly. As the cytotoxicity of the bonded sample improves from a grade 4 (severe reactivity) to a grade 0 (no reactivity) indicating the progression of the curing process, the AssureCure output indicates a concurrent progression of the curing process. This inverse correlation offers device manufacturers assurance that their bonded assemblies have achieved an acceptable level of cure and cytotoxic response.
|
Figure 3 shows the correlation between cytotoxicity and AssureCure detector response. |
Once integrated into a production line, the light cure adhesive system provides a reliable and quantitative measurement of the degree of cure for each part produced. The data obtained from the system shows an excellent correlation to percent conversion using FTIR analysis, performance properties, and biocompatibility compliance. The system also provides manufacturers a rapid and immediate quality check on 100% of all parts produced. This in-line quality assessment eliminates the buildup of defective product inventory waiting for off line quality checks.
Medical Applications
In-line light cure adhesive systems such as AssureCure technology has been integrated into high-volume medical device assembly applications where complete cure of the adhesive is critical. In such applications, the adhesive must meet strict ISO 10993 biocompatibility requirements.
|
Application #1: Safety Needle. Production for a new safety needle device relied upon a light-curing adhesive to secure a cannula into a polycarbonate hub. Earlier designs of the same device employed fluorescent adhesives in-line to ensure that adhesive was placed in the right locations. To guarantee the completeness of cure, a percentage of all assemblies were removed from the production line to validate the quality of the bond via a pull test. This off-line testing sacrificed assemblies, required additional resources for testing, and failed to prevent the buildup of defective parts when problems arose. Because the device manufacturer wanted a more efficient, in-line way to validate the integrity of bonded joints, they incorporated AssureCure technology into the existing footprint for the production process. It eliminated off-line testing, inspected 100% of assemblies, and verified the integrity of the cannula-to-hub assembly.
Application #2: Drug Delivery Device. In an application plagued with costly rework, the complex cylindrical design of a drug delivery device resulted in undercured light cure acrylic adhesive whenever parts were not accurately aligned. Although fluorescence was used to validate that the adhesive was placed in the appropriate location on the device, there was no way to confirm correct alignment and complete cure. Validation of parts alignment and adhesive cure could only take place off-line well after the device was assembled. This issue produced an inventory of defective parts that had to be reworked or scrapped. Upon introducing AssureCure adhesive during parts assembly and the detection system immediately following the cure process, 100% of the devices were inspected and defective assemblies were immediately identified. This saved the manufacturer the expense of costly components and wasted assembly processes.
Conclusion
Using a system such as the AssureCure in-line degree-of-cure technology ensures that each medical device assembly produced has been exposed to light of the appropriate wavelength and intensity, resulting in consistent high-quality devices. Adding such a system to a high-speed production line can reduce or eliminate costly off-line quality control tests such as analytical degree-of-cure testing or destructive physical testing. The output from the system correlates to important properties of the cured adhesive including cytotoxicity, which relates directly to the safety of the device. The in-line degree-of-cure technology is ideally suited for high speed or high-reliability production processes.
Anne Forcum is medical application engineer at Henkel Corp. (Rocky Hill, CT). Christine Marotta is medical segment manager and John Lafond is technology manager at the company.
About the Author(s)
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)
