Isn’t it Time for a Better IV Pole?: Adventures in Medical-Device Usability
This is a story about usability that has nothing to do with regulation and nothing to do, at least initially, with human factors professionals. The story begins with a patient—Cari Ugent, who was in the unenviable position of spending a few weeks in the hospital connected to an IV pole. If you’re ambulatory in the hospital, which Cari was, and if you’re on an IV, which she also was, then it’s not just a matter of lying there next to the pole; you have to bring it along with you wherever you go.
June 29, 2012
This is a story about usability that has nothing to do with regulation and nothing to do, at least initially, with human factors professionals. The story begins with a patient—Cari Ugent, who was in the unenviable position of spending a few weeks in the hospital connected to an IV pole. If you’re ambulatory in the hospital, which Cari was, and if you’re on an IV, which she also was, then it’s not just a matter of lying there next to the pole; you have to bring it along with you wherever you go. During her ordeal, Cari, being a writer and journalist, and a naturally curious person, began to wonder why her new intimate friend, the IV pole, was so terrible—for example, why she kept injuring her feet on the legs of the pole, why the wheels got hung up on door thresholds, why it was so awkward and top-heavy, why the lines and cords kept getting entangled in the wheels, and why it was so ugly and intimidating-looking.
|
Stephen B. Wilcox, Ph.D., FIDSA |
Then the clincher—when she commented on how difficult it was to disconnect all the pumps (which were all separately plugged into wall sockets) in order to go to the restroom, one of the nurses suggested that she should simply switch to a bedpan.
At that point, Cari put her journalist hat on and started interviewing the healthcare professionals (HCPs) in the vicinity about IV poles. She quickly learned that she wasn’t, in fact, crazy to think that her IV pole was somewhat short of perfect—she got an earful. The nurses and techs told her about how the legs of IV poles damage walls and furniture, about how there are too few hooks and too little pole space, about how intimidating they are for children, etc., etc.
Cari was certainly not the first patient who ever had to contend with an IV pole. No doubt, billions of patients had already gone through some version of what she went through, with many of the same complaints. What was different about Cari, though, was her reaction to the situation. She decided to go into the IV-pole business. She decided to go into the IV-pole business knowing little or nothing about the medical device industry or even about product development, in general, let alone about “user-centered design” or human factors. And, no, she didn’t even have a pile of cash to invest.
Read all Adventures in Medical Device Usability by Steve Wilcox |
But she didn’t really think of starting a company at first. She began by creating sketches of possible IV poles, which she showed to the HCPs in the vicinity, refining her sketches on the basis of the feedback she received. It became a way of occupying her time during her long stay in the hospital. Then, when she was discharged, she continued to work on it, and brought her latest sketches with her on the regular visits that followed her discharge.
Before long, she had at least the beginnings of a user-centered design. In fact, she had stumbled onto a seat-of-the-pants version of the design approach that is typical these days for medical devices and is advocated by the FDA.
What she did next was to take her sketches to a design firm, TEAMS Design, a firm with offices in Chicago, as well as in Europe and Asia. Cari managed to find TEAMS, which turns out to be quite a good firm, by searching on the internet without any particular prior knowledge of the field. TEAMS listened to what Cari had to say and then did some research of their own—visiting a number of hospitals to observe IV poles in use and to discuss them with users. From this work (what is called “contextual inquiry” in FDA’s Draft Guidance, Applying Human Factors and Usability Engineering to Optimize Medical Device Design, 6/22/11), they confirmed that the problems Cari had identified were by no means limited to her particular hospital. And they found some additional problems of their own and generally learned about what an IV pole has to do, where it’s used, what gets damaged in the field, and so on.
 The next step was to come up with a new IV pole design that solved the various problems, as much as possible, while still staying within very tight cost constraints. Let’s face it; nobody’s about to pay $10,000 for an IV pole, even for a perfect IV pole. TEAMS came up with a design and produced a working prototype. One interesting footnote is that TEAMS addressed the functional issues that started Cari on her journey, but they also brought her kicking and screaming to see that other things, like aesthetics, were also important.
The next step was to come up with a new IV pole design that solved the various problems, as much as possible, while still staying within very tight cost constraints. Let’s face it; nobody’s about to pay $10,000 for an IV pole, even for a perfect IV pole. TEAMS came up with a design and produced a working prototype. One interesting footnote is that TEAMS addressed the functional issues that started Cari on her journey, but they also brought her kicking and screaming to see that other things, like aesthetics, were also important.
At this point, Cari thought it was time to widen her scope, because, so far, she had relied, for her own feedback, on the one hospital where she had been a patient. In other words, she wanted to make sure that she didn’t have a design that was optimized for a single hospital. So she took it to the University of Chicago Medical Center to see what they thought.
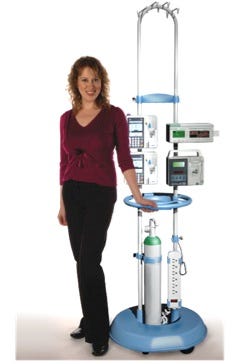
What they thought was that they wanted 100 of the poles. Meeting this first order, which also served, in effect, as a “Beta Test”, was how Cari got into the IV-pole business. Her product is called Safepole, shown in the image along with Cari Ugent herself.
As you can see in the image, Safepole improves upon the traditional IV pole in a number of ways. It has enough hooks—8—to reflect the expansion in the number of drips that many patients receive today (unlike in the 1940s, when the typical IV pole design emerged). It has 2 telescoping poles instead of one, so holds more pumps. The device above the pumps is a router that helps to organize the IV lines. Safepole has storage space for an oxygen tank, hooks for Foley catheters, and a built-in power strip. The legs are covered to reduce injuries to patients’ feet, and the cover has a bumper to reduce damage to walls and furniture. It has large, 3 in. wheels, so is less prone to getting stuck on irregularities in the floor. It has a handle (in Cari’s hand in the image) and a utility tray built in (adjacent to the handle).
In other words, Safepole is a dramatically improved product, one that successfully solves many of the problems with the traditional IV pole.
I find the Safepole story interesting for a couple of reasons. First of all, since FDA doesn’t regulate IV poles, it shows that the same methods advocated and required by FDA—contextual inquiry and usability testing—yield good products. These methods are not just about regulation, per se. With everyone scrambling right now to meet human-factors-related regulatory requirements, I fear that we sometimes lose sight of the fact that the regulatory requirements are not just ends in themselves, but are designed to assure good safe medical devices.
Another interesting aspect of the Safepole story is that the key person behind it, Cari Ugent, was not only not trained in human factors, she had no background in anything having to do with developing a medical device. She was a patient who was unhappy with her device and decided to do something about it. No doubt, an advantage she had was that, unlike most of the people reading this, she didn’t know a thousand reasons why she couldn’t just develop her own IV pole, one that, by the way, was the winner of a Gold Award in this year’s Medical Device Excellence Awards in the General Hospital Devices and Therapeutic Products category. In fact, I will reveal, as a member of this year’s jury, that Safepole came damn close to receiving the award for “Best in Show”.
In the meantime, Safepole is in 50 hospitals around the world, and Safepole, LLC has recently been awarded a contract with the GSA.
Not bad for a medical device developed by a hospitalized journalist without a nest egg.
By the way, you can get your very own Safepole from Safepole, LLC (www.safepole.net). And, Cari tells me that she’ll listen if you want to talk to her about mergers and acquisitions. She tells me she would even come along with the deal, and she’s got a drawer full of solutions to other annoying problems with medical devices. I guess this user-centered design business gets in your blood.
+++++++
Stephen B. Wilcox, is a principal and the founder of Design Science (Philadelphia), a 25-person firm that specializes in optimizing the human interface of products—particularly medical devices. Wilcox is a member of the Industrial Designers Society of America’s (IDSA) Academy of Fellows. He has served as a vice president and member of the IDSA Board of Directors, and for several years was chair of the IDSA Human Factors Professional Interest Section. He also serves on the human engineering committee of the Association for the Advancement of Medical Instrumentation (AAMI), which has produced the HE 74 and HE 75 Human Factors standards for medical devices.
You May Also Like


.png?width=300&auto=webp&quality=80&disable=upscale)
