Filters and IV Components
November 9, 2009
Originally Published MPMN November/December 2009
SPOTLIGHT
Filters and IV Components
Swabbable stopcock Protecting against bacteria, a swabbable stopcock is coated with a medical silver-ion-based antimicrobial technology that is effective against the most prevalent sources of catheter-related blood stream infections, according to the manufacturer. The TripleSafe swabbable antimicrobial stopcock features three layers of protection: a closed system that is designed to prevent contaminants’ penetration into the stopcock fluid path, thereby reducing the risk of blood stream infections; silver ions to reduce and prevent bacterial colonization on the product; and a flushing channel that reduces the residual volume and minimizes dead space, further helping to prevent bacterial colonization.
Protecting against bacteria, a swabbable stopcock is coated with a medical silver-ion-based antimicrobial technology that is effective against the most prevalent sources of catheter-related blood stream infections, according to the manufacturer. The TripleSafe swabbable antimicrobial stopcock features three layers of protection: a closed system that is designed to prevent contaminants’ penetration into the stopcock fluid path, thereby reducing the risk of blood stream infections; silver ions to reduce and prevent bacterial colonization on the product; and a flushing channel that reduces the residual volume and minimizes dead space, further helping to prevent bacterial colonization.
Elcam Medical Inc.
Hackensack, NJ
www.elcammedical
Epidural filter Featuring a low-profile design that promotes patient comfort and protection, an epidural filter has male and female ISO–certified luer locks that increase connection security and a housing rated to withstand a minimum pressure of 150 psig. Because typical epidural infusions can last from two to three days, the 0.2-µm bacterial-retentive polyethersulfone membrane allows for up to 96 hours of continuous use. The company offers a range of other filtration and fluid control products for infusion therapy, parenteral therapy, enteral infusion, drug-delivery, and drug-transfer applications.
Featuring a low-profile design that promotes patient comfort and protection, an epidural filter has male and female ISO–certified luer locks that increase connection security and a housing rated to withstand a minimum pressure of 150 psig. Because typical epidural infusions can last from two to three days, the 0.2-µm bacterial-retentive polyethersulfone membrane allows for up to 96 hours of continuous use. The company offers a range of other filtration and fluid control products for infusion therapy, parenteral therapy, enteral infusion, drug-delivery, and drug-transfer applications.
Filtertek Inc.
Hebron, IL
www.filtertek.com
Double check valves A series of double check valves is designed for transferring fluid from a clean supply vessel connected to the valve supply port (chimney) using a luer connection or tubing. The fluid is withdrawn from the supply vessel by means of a syringe connected to the aspiration port. When the syringe is compressed, the fluid is transferred through the exit port without adulterating the fluid. Providing high flow rates, the bidirectional double check valve has two inlet low-cracking pressure ranges, two supply-port options, and two exit-port options. There is no need to disconnect the valve to fill the syringe. DCV-series double check valves are manufactured entirely from materials that comply with USP Class VI criteria and are compatible with EtO, gamma, and E-beam sterilization procedures. Complementary luer tapers comply with the requirements of ISO standard 594-1. Typical applications include wound irrigation, interventional cardiology, and radiology procedures.
A series of double check valves is designed for transferring fluid from a clean supply vessel connected to the valve supply port (chimney) using a luer connection or tubing. The fluid is withdrawn from the supply vessel by means of a syringe connected to the aspiration port. When the syringe is compressed, the fluid is transferred through the exit port without adulterating the fluid. Providing high flow rates, the bidirectional double check valve has two inlet low-cracking pressure ranges, two supply-port options, and two exit-port options. There is no need to disconnect the valve to fill the syringe. DCV-series double check valves are manufactured entirely from materials that comply with USP Class VI criteria and are compatible with EtO, gamma, and E-beam sterilization procedures. Complementary luer tapers comply with the requirements of ISO standard 594-1. Typical applications include wound irrigation, interventional cardiology, and radiology procedures.
Value Plastics Inc.
Fort Collins, CO
www.valueplastics.com
Needleless access devices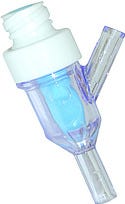 Needleless access devices (NACs) feature a patented technology ensuring that the access port of the device is flat, smooth, and free of nooks and crannies. Incorporating Tru-Swab technology, the NACs’ Y-sites will not harbor bacteria. Their double-sealed barrier design guarantees that the valve will always return to its original position, providing a surface for friction swabbing during preaccess disinfection. The components’ design aids in the reduction of blood stream infections, according to the manufacturer, and their clear housing provides a visual reminder to healthcare personnel that they must perform clinical practices such as priming, disinfection, and flushing.
Needleless access devices (NACs) feature a patented technology ensuring that the access port of the device is flat, smooth, and free of nooks and crannies. Incorporating Tru-Swab technology, the NACs’ Y-sites will not harbor bacteria. Their double-sealed barrier design guarantees that the valve will always return to its original position, providing a surface for friction swabbing during preaccess disinfection. The components’ design aids in the reduction of blood stream infections, according to the manufacturer, and their clear housing provides a visual reminder to healthcare personnel that they must perform clinical practices such as priming, disinfection, and flushing.
Medegen Inc.
Ontario, CA
www.medegen.com
Silicone peristaltic-pump tubing Peristaltic-pump tubing is made from silicone in tight-tolerance extrusion applications. Manufactured from either platinum or peroxide-cured silicones for different applications, the tubing can maintain ±0.0005-in. ID tolerances, resulting in pumping-device accuracy. Suitable for IV drug-delivery applications because they are physiologically inert, the tubing does not contain plasticizers that can leach out and contaminate the mixture being pumped. They can also be sterilized using a range of methods common to the medical device manufacturing industry.
Peristaltic-pump tubing is made from silicone in tight-tolerance extrusion applications. Manufactured from either platinum or peroxide-cured silicones for different applications, the tubing can maintain ±0.0005-in. ID tolerances, resulting in pumping-device accuracy. Suitable for IV drug-delivery applications because they are physiologically inert, the tubing does not contain plasticizers that can leach out and contaminate the mixture being pumped. They can also be sterilized using a range of methods common to the medical device manufacturing industry.
Specialty Silicone Fabricators
Paso Robles, CA
www.ssfab.com
For more features and articles on filters and IV components, go to devicelink.com/mpmn/ivfilter
Copyright ©2009 Medical Product Manufacturing News
You May Also Like


