Designing a Device for Drug Delivery? What You Need to Know about Extractables, Leachables, and Particles
New expectations are evolving for particles and extractables and leachables.
August 17, 2017
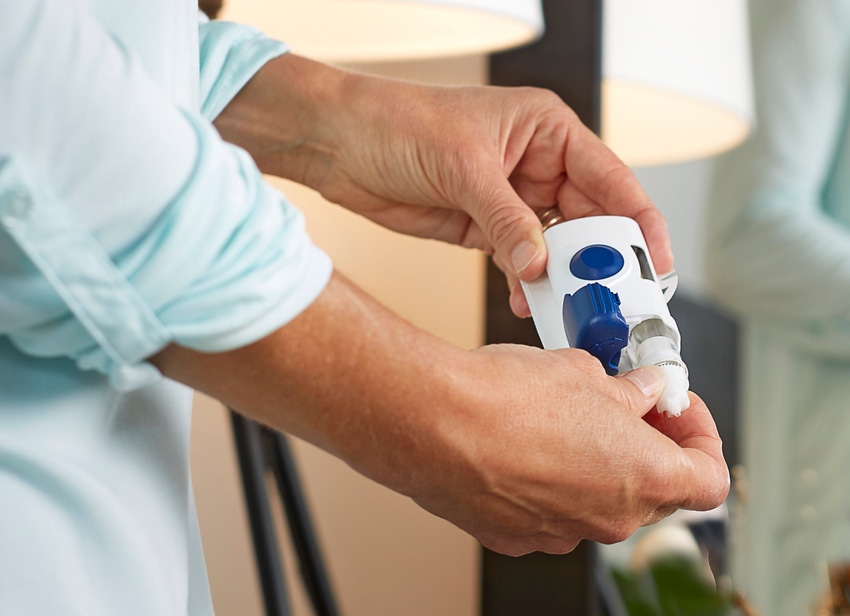
Growing demand for drug-delivery devices is bringing the pharmaceutical and medical device industries closer together. The risks posed by particulates and extractables and leachables, for instance, present challenges for both industries, so drug-delivery device designers and their pharma partners are encouraged to take a risk-based approach to testing. Standards bodies and industry groups are already working on guidances to help, but much of the critical thinking is up to the manufacturers themselves.
"One trend we're seeing is the intimate combination of drug/package development with how the drug is delivered to the patient," explained Fran DeGrazio, vice president of scientific affairs and technical services at West Pharmaceutical Services Inc., to Qmed. "Historically, there have been different standards." And there have been different regulatory expectations, she added, pointing to CFR Parts 210 and 211 guiding drug makers and CFR Part 820 guiding device manufacturers.
"But when you start down the path of drug-delivery device development, you need to think about both," she advised.
Options for drug delivery today include prefilled syringes, IV container systems, autoinjectors, wearable systems, and more, she said.
One of the critical topics today is particulate contamination. For instance, a new PDA task force is looking to set guidance on achieving "zero visible particles" in the final drug format, says DeGrazio. "The team is focused on multiple aspects of the fill/finish process and is currently focused on glass and elastomer primary components."
West is leading a subteam on the task force that is working to optimize an analytical method for visible particles on elastomeric closures, DeGrazio added. "This method has previously been used to analyze particulates from elastomer components and is being refined with input from other pharma and supplier representatives to build a standard for the industry," she explained. PDA is expected to hold workshops in 2018 to discuss the ongoing taskforce activities and related deliverables.
USP has also announced that they will kick off a team to work on particulate and various elastomer, glass, and plastic materials, she said.
Another area of high industry activity is extractables and leachables approaches. Diane Paskiet, director of scientific affairs for West, is involved with USP to modernize chapters associated with packaging, leachables, and extractables (L&E); she is also leading the Product Quality Research Institute (PQRI) leachables and extractables working group to provide recommendations on threshold and best practices for L&E in parenteral and ophthalmic drug products (PODP). The outcome of the working group, associated workshop presentations and publications can be found at www.pqri.org.
When it comes to testing for particles or extractables and leachables, regulators are encouraging a "risk-based approach" and the use of "critical thinking," said DeGrazio. "This means an approach that is logical, based on good science and reasoning specific to the unique drug and its application versus an historic approach where everything is tested the same way no matter what.
"The approach should be based on what is appropriate for a given product," she continues. "If there's a particle, what is it? Could it be filtered out? What risks does it present? Components from a delivery system should also be characterized for extractables, especially critical componentry that comes into direct contact with the patient or drug product. Concerning leachables, safety understanding must focus on the patient population, therapeutic area, and other pertinent aspects of the drug product application.
"It is all about managing risk," DeGrazio added. "Prioritize what is being evaluated through a critical thinking approach--don't just follow a routine series of tests. Are the choices being made logical? Think about the end first--how is the product going to be used, how will it touch the patient? Is it injected, and how often? Will it be used on pediatric patients, or healthy adults? A leachable for a sick child could be a significant issue."
Much of the recent compendial changes in USP chapters, such as those involving plastics, glass, and elastomers, do encourage such "critical thinking," she said. "USP chapters will aid with selection of materials, but you need to justify an appropriate approach for your specific product. Is it ultimately the right material for your product?"
In addition to having its team members participate in industry working groups, West works to help its customers understand these changing expectations and develop the right approach for their drug products.
Don't miss these sessions and more at the upcoming MD&M Minneapolis Conference and Expo, November 8-9, 2017:
"How Usability Research & Engineering Are Changing Medical Device Development," Speakers: Sean Hägen (BlackHägen Design), Michael Lynch (Intertek)
"Case Study: How Human-Centered Design Disrupted Cancer Treatment," Speaker: Mu Young Lee (Varian Medical Systems)
"From Inception to Validation: A Comprehensive Approach to Optimizing Usability While Meeting FDA Requirements," Speaker: Natalie Abts (MedStar Health, National Center for Human Factors in Healthcare)
"Case Study: How In-Home Use Revolutionizes the Usability of Medical Devices," Speaker: Russ Branaghan, PhD (Research Collective)
Daphne Allen is executive editor of Pharmaceutical & Medical Packaging News and a contributor to MD&DI and Qmed. Reach her at [email protected] and on Twitter at @daphneallen
[Image credit: West Pharmaceutical Services Inc.]
About the Author(s)
You May Also Like



.png?width=300&auto=webp&quality=80&disable=upscale)
