Could This Device Replace Drugs for Crohn's Treatment?
October 20, 2016
SetPoint Medical's vagus nerve stimulation implant has shown promise for treating the debilitating inflammatory bowel disease, according to a new European study.
Nancy Crotti
|
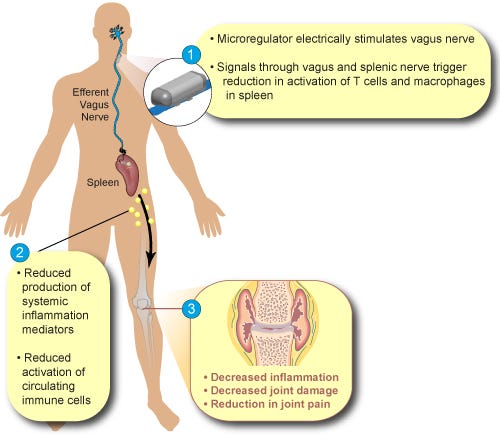
SetPoint Medical is also studying its implantable neuromodulation technology for treatment of rheumatoid arthritis. Image courtesy of SetPoint Medical |
A European study using a bioelectronic device has shown positive results in treating Crohn's Disease.
The implant, made by SetPoint Medical of Valencia, CA, delivers timed, digital doses of electricity to stimulate the vagus nerve, activating the body's natural inflammatory reflex to produce a systemic anti-inflammatory effect, according to a company statement. The findings were presented during United European Gastroenterology Week (UEGW) in Vienna.
A debilitating disease caused by inflammation in the digestive tract, Crohn's is often treated with anti-inflammatory or immunosuppressant drugs, which may yield unpleasant or even dangerous side effects. Crohn's may affect as many as 780,000 people in the U.S. and can occur at any age but is more prevalent among adolescents and young adults, according to the Crohn's & Colitis Foundation of America.
The open-label pilot study is being conducted at five centers in Europe, including patients with moderately-to-severely active Crohn's despite treatment with a tumor necrosis factor (TNF) antagonist drug.
In this study, eight patients with severe Crohn's Disease that is not responsive to TNF antagonists received an implant on the vagus nerve in their neck to deliver the electric stimulation. Improvement was assessed at the 16-week mark using the Crohn's Disease Activity Index (CDAI), a tool used to quantify the symptoms of patients with the disease. Severe Crohn's is defined by a CDAI greater than 450, and remission is defined as a CDAI below 150.
The CDAI scores of six of the eight patients in the study were reduced by 70 points or more, and CDAI remission and endoscopic remission were achieved in three patients. The data also show a direct correlation between vagus nerve stimulation and the suppression of proinflammatory cytokines, including TNF, which plays a key role in inflammation, a finding consistent with the results from an earlier company study in patients with rheumatoid arthritis.
"We are very encouraged by these initial positive results using bioelectronic medicine to treat Crohn's Disease," said Geert D'Haens, M.D., professor of gastroenterology at The Academic Medical Center (AMC), University of Amsterdam and coordinating investigator for the study. "The treatment was well tolerated and significantly reduced Crohn's symptoms in six of eight patients. We are evaluating a new cohort of patients as the study continues."
SetPoint's approach is designed to supplement the body's natural inflammatory reflex by providing "built-in" therapy, and is intended to improve safety compared with drugs or biologic solutions. The company has attracted investments from Boston Scientific, Covidien and Action Potential Venture Capital.
Nancy Crotti is a is a contributor to Qmed.
Like what you're reading? Subscribe to our daily e-newsletter.
About the Author(s)
You May Also Like

.png?width=300&auto=webp&quality=80&disable=upscale)
